Abstract
n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA), from fish oil (FO), in rodents have been shown to reduce adipogenesis. Evidence of an effect on adipose tissue mass in humans is limited, and no studies have specifically aimed to elucidate this in infancy. To explore whether n-3 LCPUFA intake affects adipose tissue growth, we randomly allocated 154 healthy infants to daily supplementation with FO or sunflower oil (SO) from 9 to 18 mo of age and measured z-score changes in various anthropometric assessments of body size and skinfold thicknesses and plasma adipokine concentrations. Among the 133 completing infants, erythrocyte n-3 PUFA increased more in those receiving FO than in infants receiving SO [12.2 ± 0.7 (mean ± SE) versus 2.0 ± 0.4 fatty acid percentage (FA%), p < 0.001] with a concomitant larger decrease in n-6 PUFA (−8.9 ± 0.7 versus −0.9 ± 0.6 FA%, p < 0.001). We found no association between FO consumption relative to SO consumption and any of the anthropometric measures related to the size of the fat mass, but infants in the FO group had a lower skinfold ratio (triceps/subscapular) at 18 mo than those in SO group (p = 0.02). Our findings do not support the hypothesis that dietary n-3 LCPUFA is important for infant fat mass, but future studies testing this specifically are warranted.
Similar content being viewed by others
Main
A sedentary lifestyle that favors a positive energy balance is generally acknowledged as the major cause of the increasing prevalence of obesity, but other potential contributing factors are also receiving attention. Among these are the dietary changes in the consumption of polyunsaturated fatty acids (PUFA) toward a higher n-6 and lower n-3 PUFA intake, which has been suggested as a partial explanatory factor (1). Dietary n-3 long-chain PUFA (LCPUFA) in rodents has been shown to prevent diet-induced obesity (2) and has also been reported to enhance fat mass reduction in two randomized weight-loss trials in overweight adults (3) and obese women with type-2 diabetes (4).
Early life is a period of rapid growth with major changes in both body size and composition. It is well established that docosahexaenoic acid (DHA, 22:6n-3) is important for, e.g., visual development in infancy (5), but it is presently unknown to what extent n-3 LCPUFA should be considered relevant with respect to adiposity. The natural content of n-3 PUFA in breast milk has been found to be positively associated with infant weight gain in preterm (6) but not term-born infants (7). Furthermore, two intervention studies giving n-3 LCPUFA to pregnant and/or lactating mothers suggest a programming effect on later infant BMI, although in opposite directions (8,9). However, no studies have to our knowledge aimed specifically to investigate whether dietary n-3 LCPUFA in early life is associated with the size of the fat mass per se.
Inspired by the clear finding in rodents, this study was conducted to attempt to elucidate whether growth and fat deposition in healthy Danish infants during the complementary feeding period, 9–18 mo of age, are associated with the dietary intake of n-3 LCPUFA. To ensure variation in the daily intake in the study population, we performed a randomization to a daily supplement of either fish oil (FO, n-3 LCPUFA) or sunflower oil (SO, n-6 PUFA). Because of the explorative nature of this study, we did not a priori have any specific hypotheses, and consequently a number of different measures related to growth and fat deposition were obtained before and after the study period. We furthermore investigated whether dietary n-3 LCPUFA was related to plasma levels of hormones produced and secreted by the adipocytes, adiponectin, and leptin.
SUBJECTS AND METHODS
Subjects and study design.
The study was a double-blinded controlled, parallel-intervention where 9-mo-old infants were randomly allocated to a daily oil supplement for a period of 9 mo. All participants were examined before and after the intervention, at 9 ± 0.5 mo and 18 ± 0.9 mo, respectively. Eligible infants were healthy singletons, born ≥37th week of gestation with appropriate weight for GA (10), 5-min Apgar score >7, no previous FO supplements, and no use of medications that may influence growth and/or food intake. Families resident in the Capital Region of Copenhagen were randomly selected from the National Danish Civil Registry and sent invitations to participate. Parents who responded to the invitation were invited to an information meeting at Department of Human Nutrition, University of Copenhagen, where they gave their written informed consent (see diagram of participant flow in Fig. 1). Detailed questionnaires were completed at baseline and when infants were 18 mo old, and parents and caretakers of the participants filled in precoded food diaries for the infants in seven consecutive days before both the examinations. Before the registration week, parents were carefully instructed on how to register their infant's diet, and one of two investigators checked all food registrations with the accompanying parent during the examination visits to increase data quality. The study was approved by the Scientific Committees of the Capital Region of Copenhagen, Denmark (H-A-2007-0088) and registered in www.Clinical-Trials.gov (NCT 00631046).
Dietary intervention, randomization, and group allocation.
The intervention consisted of a daily 5 mL oil supplement from 9 to 18 mo of age of either FO [rich in eicosapentaenoic acid (20:5n-3, EPA) and DHA but without vitamins] or SO [rich in linoleic acid, (18:2n-6)] (a kind gift from Axellus A/S). We chose SO as the control because we wanted to investigate the specific effects of n-3 PUFA. The intended dose would supply infants in the FO group with 1.6 g/d EPA + DHA and those in the SO group with 3.1 g/d linoleic acid. Because of the additional energy in the supplements, parents were instructed to substitute some of the usual fat in their infant's diet with the supplied oil and to give the oils with a spoon or mixed with foods. The instructions specified that all unopened bottles should be kept in the freezer and refrigerated once opened to minimize fatty acid oxidation.
All 154 enrolled infants had an equal probability of allocation to the two groups. A person not involved in the study made the randomization list by computer-generated block randomization with varying block sizes of 4, 6, 8, and 10 in randomly order (www.randomization.com). Hence, ID numbers 1–170 were assigned to one of the two intervention groups. Ten dark plastic 150-mL bottles containing the intervention oils were subsequently coded and labeled with each ID number. The investigators responsible for the contact with the infants and families allocated the next available number from the list on the next entry into the study, and the 10 intervention oil bottles (enough for the entire intervention period) were provided after the baseline examination. Investigators were at all times blinded to the allocation, and unblinding was performed only after completing all data analyses. The randomization list was kept in a sealed envelope in a safety box at the department.
Adherence to the oil intervention.
The parents were requested to return all 10 bottles at the 18-mo examination and to report spilling of oil, etc. Compliance in 131 of the 133 completing infants was estimated from the original weight of 10 full bottles and the returned bottles including leftovers plus the exact number of days a given participant had been in the study. An estimate of the provided amount of PUFA from the supplements was calculated from the mean weight% of the individual fatty acids obtained from two separate fatty acid analyses of the intervention oils. Compliance was furthermore assessed from the erythrocyte (RBC) PUFA composition, a biomarker of long-term PUFA intake (11), which was determined in the participants before and after the intervention.
Study outcomes.
The study was primarily undertaken to compare changes in anthropometric measurements in the FO group relative to the SO group. Only three researchers, including the principal investigator, were involved in the measuring process. At each of the two examinations, a series of standardized anthropometric measurements were performed, according to Gibson (12), by two trained investigators in the presence of the infant's parents. All anthropometric measures, except weight, were obtained in three replicates, and the mean values of these were used in analyses. The recumbent length of the infants was measured to the nearest millimeter on a measuring board (Force Technology, Brøndby, Denmark). Naked weight was measured sitting or lying down on a pediatric infant scale (Sartorius IP65; Bie & Berntsen, Rødovre), which took 40 consecutive weights in 10 s and displayed the mean. If the infant weighed >12 kg, an adult scale was used (Lindeltronic 8000; Samhall Lavi AB, Kristianstad, Sweden). BMI was calculated as weight (kg)/length (m2). Head circumference and mid-upper-arm circumference were measured to the nearest millimeter with a paper measuring tape (Lasso, Child Growth Foundation, London, United Kingdom). Triceps and subscapular skinfold thicknesses were measured to the nearest 0.1 mm (Harpenden skinfold caliper; CMS Weighing Equipment, Ltd., London, United Kingdom). The interobserver variability for triceps skinfolds measurements was <0.5 mm, which shows good reliability (13).
Sex- and age-standardized z-scores for weight-for-age, length-for-age, BMI-for-age, weight-for-length, head circumference-for-age, mid-upper-arm circumference-for-age, triceps skinfold-for-age, and subscapular skinfold-for-age at 9 and 18 mo were calculated using macros from the WHO-Anthro webpage (http://www.who.int/childgrowth/software/en/). We also calculated the ratio triceps skinfold (mm)/subscapular skinfold (mm).
Measures of secondary interest were group changes in plasma adipokine concentrations. Parents were instructed to keep their child from eating >2 h before blood sampling at both examinations. The final mean fasting time was not different in the two groups, being 157 ± 46 (±SD) and 150 ± 33 min at 9 and 18 mo, respectively. At the end of both examinations, 6-mL blood samples were taken by venipuncture, kept on ice and separated into plasma, buffy-coat, and RBC by centrifugation at 2300 × g for 10 min at 4°C. Plasma for analysis of adiponectin and leptin was taken from EDTA-coated tubes and stored at −80°C until analysis. Analyses were performed according to the manufacturer's instructions with RIA kits for human leptin (CAT.#HL-81 K; Millipore, MO) and human adiponectin (CAT.#HADP-61HK; Millipore; measuring all multimeric forms but not the monomeric form or globular domain). Plasma samples were thawed, kept on ice, and analyzed in duplicates, and accepted coefficients of variation (CV%) in the two analyses were <10% and <15%, respectively. IGF-1 concentrations were assessed in heparin plasma in accordance with the manufacturer's instructions by automated chemiluminescent immunoassay (Immulite 1000; DPC Biermann GmbH, Bad Nauheim, Germany) and kits for human IGF-1 (LKGF1; Siemens, LA).
The fatty acid composition of RBC from heparin-coated tubes was determined as previously described (14). Values are expressed as area% of the specific fatty acid relative to the total chromatogram area (FA%).
Statistical analysis.
Because this study is the first of its kind, we did not have proper assessments of the predicted effect size on the anthropometric end points. Based on previous experience, we expected a drop-out rate of 10–15%, thus, by recruiting 150 infants, we expected 130 completers. With a power of 0.80 and α = 0.05, this would allow us to detect group differences of around 0.5 × SD, which is equivalent to 0.9 and 0.6 mm differences in the changes in the triceps and subscapular skinfold thicknesses, respectively. According to the WHO reference charts, this is equivalent to the normal reduction in skinfolds from 9 to 18 mo of age and is consequently considered to be of clinical relevance.
All data were checked for normality. Descriptive continuous variables are expressed as means ± SD or medians (25th–75th) as appropriate. Outcome variables are expressed as means ± SE unless otherwise noted. All statistical tests were performed using Stata 11.0 (Stata Institute, TX) and significance established at p < 0.05. Baseline characteristics between groups were compared using independent t test, Mann-Whitney U test, and χ2 test. Analysis of covariance (ANCOVA) was used to adjust for the slightly uneven sex distribution in the two groups. The habitual diet and RBC fatty acid composition of the groups at 9 and 18 mo and Δ values in RBC fatty acids were compared by independent t test, Mann-Whitney U test, and χ2 test. Within-group changes from 9 to 18 mo were evaluated using paired t test or Wilcoxon signed-rank test as appropriate. Ordinary bivariate correlations were used to explore associations between oil consumption and RBC changes in n-3 PUFA content in the FO group.
All primary analyses were complete case analyses involving all participants who were randomly assigned to one of the intervention groups and completed the study. ANOVA analysis showed a significant impact of the baseline value on the 9–18 mo change for both the anthropometric measurements and the plasma concentrations of adipokines. Thus, changes in both primary and secondary end points are analyzed using ANCOVAs adjusted for baseline values of the evaluated outcome, and analyses of skinfold ratio were also adjusted for gender. All models with z-scores are per definition adjusted for gender and exact age. The p values adjusted for baseline, plasma levels of IGF-1, and breastfeeding were also calculated, as breastfeeding is known to affect infant growth patterns. These adjusted models also analyzed the effect of the amount of oil consumed (compliance). Leptin values were log transformed before all analyses.
Dose–response analyses based on n-3 PUFA-content in RBC were conducted in infants with successful blood sampling at both 9 and 18 mo (n = 115). This was done by substituting “intervention group” in the complete case ANCOVA models with the observed changes in RBC n-3 PUFA and also explored in bivariate correlations. Finally, multiple linear regressions were used to retrieve the parameter estimates of oil consumption on skinfold thicknesses. Normality of the residuals of all final models was confirmed.
RESULTS
Participants were recruited from December 2007 to May 2008. Baseline and outcome examinations were conducted from January to June 2008 and October 2008 to March 2009, respectively. In the FO group, 14 infants left the study during the intervention and seven in the SO group. Half of the drop-outs in the FO group were reported to be due to the intervention oil, but there was no difference in attrition rate between groups.
The baseline characteristics of the groups were similar (Table 1). There were no group differences in paternal BMI [24.4 ± 2.7 (mean ± SD) versus 24.6 ± 2.7 kg/m2 in the FO and SO groups, respectively] or maternal BMI (23.1 ± 3.4 versus 23.5 ± 3.6 kg/m2, respectively), and their educational level was also similar in the groups (data not shown).
The infants had a median intervention oil intake of 3.8 (3.2–4.2) g/d, and although in absolute numbers the FO group had consumed slightly less than the SO group (Table 2), this was not statistically significant (p = 0.17). Based on the mean body weight at 18 mo (11.5 kg in both groups), the supplements provided around 3.5% of the infants' mean estimated energy requirements. The estimated daily intakes of specific fatty acids from the supplements are presented in Table 2. Because approximately 41% of the FO consisted of n-3 LCPUFA (34% EPA + DHA) and 67% of the SO of LA, the added n-3 PUFA accounted for 1.4% of total energy intake (E%) in the FO group, whereas the added n-6 PUFA accounted for 2.4 E% in the SO group.
The infants in FO group had a slightly higher PUFA intake at 9 mo of age than the SO group (Table 3). Furthermore, significantly more infants in the FO group had been introduced to fish, and the FO group also tended to have a higher fish intake (p = 0.12). The daily intake of n-3 PUFA was 0.8 and 0.7 g/d in the FO group and SO group, respectively. By the end of the intervention, energy from fat had generally been decreased and substituted with energy from carbohydrates and protein, the latter slightly lower in the FO group compared with the SO group. Fish consumption increased in the SO group during the intervention and was around 10 g/d at 18 mo of age in both groups. The absolute intake of n-3 PUFA had increased relative to baseline and was 2.4 and 0.9 g/d in the FO group and SO group, respectively. Dietary fat intake had generally increased and was similar in the two groups, suggesting that parents in both intervention groups had equal success in substituting part of the habitual fat with the supplement oils. PUFA intake from the habitual diet decreased from 9 to 18 mo and was surprisingly low compared with recommendations (15), even after addition of the supplements. At 18 mo, diet and supplements provided ∼2 E% from n-3 PUFA to the infants in the FO group and 5.5 E% from n-6 PUFA in the SO group. The estimated total PUFA intake was 5.4 and 6.5 E%, and the overall n-6/n-3 PUFA ratio was 1.6 and 7.9 in the FO group and SO group, respectively.
Changes in fatty acid composition of infant erythrocytes.
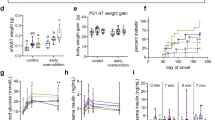
The small group difference in PUFA intake at 9 mo was not reflected in infant RBC, where the SO group had a somewhat higher PUFA content (p = 0.06). The estimated intake difference could be due to uncertainties in the Danish Food Composition Databank. The n-3 PUFA content of RBC increased during the intervention in both groups but significantly more in the FO-supplemented infants compared with that in the SO group (Table 4). The estimated mean difference in the change in RBC content of total n-3 PUFA was 10.16 (95%CI; 8.63–11.69) FA%, and the change in RBC-EPA in the FO group correlated well with the oil consumption (r = 0.6, p < 0.001). The RBC content of n-6 PUFA decreased in the FO group compared with the SO group, with a total difference between groups of 7.94 (6.09–9.79) FA%. After the intervention, infants had a median arachidonic acid-EPA ratio of 1.9 (interquartile range; 1.5–3.2) and 15.0 (10.7–19.4) in the FO group and SO group, respectively. Besides these marked changes in the RBC content of specific PUFA, minor group differences in the 9–18 mo changes in both monounsaturated fatty acids and PUFA were also observed, with estimated differences of 1.55 (95% CI; 0.59–2.51) FA% and 2.23 (0.21–4.25) FA%, respectively.
Study outcomes.
The z-scores on anthropometric assessments at 9 mo, 18 mo, and changes from 9 to 18 mo are presented in Table 5. Generally, the study population was bigger and grew more than the WHO reference population, except for the linear growth where the z-score changes were negative in both groups. Overall, the ANCOVAs adjusted for baseline showed no group differences, although children in the FO group tended to have smaller changes in z-scores for triceps skinfold thicknesses (p = 0.07). Within-group changes in most of the adiposity-related z-scores increased significantly in the SO group, and although infants in the FO group had a similar growth pattern, they did not display a significant upward movement in the z-scores. Curiously, there was a clear group difference in changes in the skinfold ratio (Table 5), which was supported by a dose-response relationship to the RBC change in n-3 PUFA (r = −0.24, p = 0.009, n = 115). Besides the associations with the skinfold ratio, substituting intervention group allocation in the statistical models with the change in n-3 PUFA content in RBC did not, in general, reveal any additional relationships between n-3 PUFA and the anthropometric measures. Adjusting the complete case models for the consumed amount of oil, breastfeeding at 9 mo (y/n), duration of full breastfeeding, and the 9–18 mo changes in IGF-1 did not change the statistical significance, but the association between triceps skinfold z-scores and oil group was weakened (Table 5). Interestingly, these models revealed that the amount of oil consumed (irrespective of PUFA type) was positively related to z-scores for both measures of skinfold thickness (Table 5). The z-scores for triceps and subscapular skinfolds increased by 0.14 (p = 0.06, R2 = 0.31) and 0.18 (p = 0.01, R2 = 0.39), respectively, for each additional milliliter/day of oil.
Plasma concentrations of leptin and adiponectin.
At 9 mo of age, plasma leptin concentrations correlated with BMI (r = 0.6, p < 0.001), triceps (r = 0.5, p < 0.001), and subscapular skinfold thicknesses (r = 0.4, p < 0.001; adjusted for current breastfeeding and duration of full breastfeeding), but no association was found between plasma adiponectin and measures of infant fat mass. The correlations between these measures and plasma leptin after adjustment for breastfeeding at 9 mo, duration of full breastfeeding, and intervention oil consumption were not as strong at 18 mo (r = 0.2, p < 0.03; r = 0.3, p = 0.005; and r = 0.4, p < 0.001 for BMI, triceps, and subscapular skinfold thicknesses, respectively). Plasma adiponectin concentrations were not related to the type of oil and stayed the same across the intervention period (Table 6). In contrast, FO consumption tended to be associated with changes in plasma leptin (p = 0.097). Although leptin concentrations remained the same in the FO group, they tended to increase slightly from baseline in the SO group (p = 0.07). However, like the association with triceps skinfolds, this association was weakened after adjusting for compliance and breastfeeding. The adjusted analysis, however, revealed that the amount rather than the type of oil was related to plasma leptin concentrations (Table 6).
DISCUSSION
This study showed no relationship between the dietary intake of n-3 LCPUFA compared with n-6 PUFA from 9 to 18 mo of age and body size (weight, length, and head circumference). The lack of such an association is generally consistent with findings from previous studies in early life (9,16,17). Qualitative changes in dietary fats with a general decrease in infant dietary n-3 PUFA have been proposed to contribute to the world-wide obesity epidemic (1). However, in spite of the raised concerns (1), data on the relationship between n-3 LCPUFA and infant adiposity are sparse and mostly limited to calculated BMI. In this first study, which specifically attempted to explore the relationship, we found no clear association with any of the anthropometric measures. Similarly, a trial comparing infant formula with and without DHA from birth to 17 wk of life observed no group differences in weight-for-length or skinfolds at any time during the first year of life (18). In line with this, we have previously found no group differences in BMI during the first year between infants of FO- or olive oil-supplemented lactating mothers (9). Surprisingly, in this previous study, we observed that BMI and skinfolds at 2½ years were higher in the FO group, indicating some sort of programming effect. Conversely, Lucia Bergmann et al. (8) found that infants of DHA-supplemented pregnant and lactating German mothers had a lower BMI at 21 mo. The German study was conducted in a population with a relatively low fish intake, which could account for some of the discrepancies, as also pointed out by Lucia Bergmann et al. (8). Although in this study we found no associations with measures indicative of the size of the fat mass, our findings indicate a possible relation with the ratio of the s.c. fat at triceps and the subscapular sites. Site-specific actions of dietary n-3 LCPUFA on different fat depots have been reported in both overweight and obese women (4) and in a recent rodent study (19).
None of the measured adipokines were associated with our intervention. In contrast to this, increasing dietary n-3 LCPUFA has been shown to increase circulating adiponectin concentrations in adults (20) and to decrease circulating levels of leptin in rodents (2). The plasma adiponectin levels at 9 mo in our study were similar to that reported by others (21) but remained at the same concentration from 9 to 18 mo. Consistent with observations in adults (22), we found that plasma leptin levels correlated positively with BMI at both 9 and 18 mo, and similar findings have also been reported by others (21,23).
Like in any other in principle double-blinded FO intervention, the study was essentially single blinded. Although the FO was odorless when kept refrigerated, it may have influenced the palatability of the foods to which it was added. This could then be speculated to have affected the food intake in the FO group, and consequently, partly mediate the observed trends in the study. This is, however, not supported by the dietary registrations at 18 mo. The marked group differences in RBC n-3 PUFA content at 18 mo could be expected to be large enough to detect any associations with fat tissue mass. One proposed mechanism is based on differences in the relative tissue amounts of arachidonic acid and EPA, because arachidonic acid has been shown in vitro (24) and in rodents (1) to promote adipose tissue development. It should be noted that n-6 PUFA have been proposed to be prolipogenic (25), and the observed trends could thus be speculated to be driven by an increased fat deposition in the SO group. However, we find that both oil supplements are positively related to s.c. fat deposition, which could imply that the parents did not completely compensate the intake of intervention oils in the habitual fat intake of their infants. If so, then the z-score increase within the SO group is probably related to excess energy intake, whereas the absence of a similar increase within the FO group could be due to the n-3 LCPUFA. In that case, our study lacked power to show the resulting group difference. However, quite a number of statistical tests were performed in this study which increases the risk of chance findings.
In conclusion, FO compared with SO consumption in healthy Danish infants from 9 to 18 mo of age was not associated with fat deposition, growth, or plasma levels of adipokines. It was, however, related with differences in the distribution of the s.c. fat depots indicating that n-3 LCPUFA consumption may influence the adipose tissue in human infants. This association along with its biological interpretation needs to be confirmed, and future studies specifically testing the hypothesis that n-3 LCPUFA can reduce infant adiposity are warranted.
Abbreviations
- ANCOVA :
-
analysis of covariance
- DHA :
-
docosahexaenoic acid
- E% :
-
percentage of total energy intake
- EPA :
-
eicosapentaenoic acid
- FA% :
-
percentage of fatty acid relative to chromatogram area
- FO :
-
fish oil
- LCPUFA :
-
long-chain PUFA
- PUFA :
-
polyunsaturated fatty acids
- SO :
-
sunflower oil
References
Ailhaud G, Guesnet P, Cunnane SC 2008 An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development?. Br J Nutr 100: 461–470
Jang IS, Hwang DY, Chae KR, Lee JE, Kim YK, Kang TS, Hwang JH, Lim CH, Huh YB, Cho JS 2003 Role of dietary fat type in the development of adiposity from dietary obesity-susceptible Sprague-Dawley rats. Br J Nutr 89: 429–438
Hill AM, Buckley JD, Murphy KJ, Howe PR 2007 Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 85: 1267–1274
Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW 2007 Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 86: 1670–1679
Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE 2003 Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. J Pediatr 143: S17–S25
Tinoco SM, Sichieri R, Setta CL, Moura AS, doCarmo MG 2009 n-3 polyunsaturated fatty acids in milk is associate to weight gain and growth in premature infants. Lipids Health Dis 8: 23
Scholtens S, Wijga AH, Smit HA, Brunekreef B, de Jongste JC, Gerritsen J, Seidell JC 2009 Long-chain polyunsaturated fatty acids in breast milk and early weight gain in breast-fed infants. Br J Nutr 101: 116–121
Lucia Bergmann R, Bergmann KE, Haschke-Becher E, Richter R, Dudenhausen JW, Barclay D, Haschke F 2007 Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy?. J Perinat Med 35: 295–300
Lauritzen L, Hoppe C, Straarup EM, Michaelsen KF 2005 Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatr Res 58: 235–242
Greisen G, Michaelsen KF 1989 [Perinatal growth. A practical perinatal growth curve]. Ugeskr Laeger 151: 1813–1815
Kuratko CN, Salem N 2009 Biomarkers of DHA status. Prostaglandins Leukot Essent Fatty Acids 81: 111–118
Gibson RS 2005 Principles of Nutritional Assessment. Oxford University Press, New York, pp 245–293
Ulijaszek SJ, Kerr DA 1999 Anthropometric measurement error and the assessment of nutritional status. Br J Nutr 82: 165–177
Lauritzen L, Jorgensen M, Mikkelsen T, Skovgaard I, Straarup EM, Olsen S, Hoy CE, Michaelsen K 2004 Maternal fish oil supplementation in lactation: effect on visual acuity and n-3 fatty acid content of infant erythrocytes. Lipids 39: 195–206
Nordic Council of Ministers 2005 Nordic Nutrition Recommendations 2004: Integrating Nutrition and Physical Activity. Norden, Copenhagen, pp 157–172
Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA 2001 Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108: E82
Makrides M, Gibson RA, Udell T, Ried K, International LCPUFA Investigators 2005 Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr 81: 1094–1101
Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C 1998 Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res 44: 201–209
Rokling-Andersen MH, Rustan AC, Wensaas AJ, Kaalhus O, Wergedahl H, Rost TH, Jensen J, Graff BA, Caesar R, Drevon CA 2009 Marine n-3 fatty acids promote size reduction of visceral adipose depots, without altering body weight and composition, in male Wistar rats fed a high-fat diet. Br J Nutr 102: 995–1006
Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y 2007 Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 27: 1918–1925
Iñiguez G, Soto N, Avila A, Salazar T, Ong K, Dunger D, Mericq V 2004 Adiponectin levels in the first two years of life in a prospective cohort: relations with weight gain, leptin levels and insulin sensitivity. J Clin Endocrinol Metab 89: 5500–5503
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, Mckee LJ, Bauer TL, Caro JF 1996 Serum immunoreactive leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295
Lönnerdal B, Havel PJ 2000 Serum leptin concentrations in infants: effects of diet, sex, and adiposity. Am J Clin Nutr 72: 484–489
Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, Guesnet P, Amri EZ, Negrel R, Ailhaud G 2003 Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern?. J Lipid Res 44: 271–279
Muhlhausler BS, Cook-Johnson R, James M, Miljkovic D, Duthoit E, Gibson R 2010 Opposing effects of omega-3 and omega-6 long chain polyunsaturated fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J Nutr Metab 2010: 927836
Acknowledgements
We thank all the participating children and their families and the biotechnicians for their technical assistance. We also thank the involved students, especially Laurine Schram, for their invaluable work with the participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Danish Council for Strategic Research, Programme Commission for Food and Health. Intervention oils donated by Axellus A/S.
Rights and permissions
About this article
Cite this article
Andersen, A., Michaelsen, K., Hellgren, L. et al. A Randomized Controlled Intervention With Fish Oil Versus Sunflower Oil From 9 to 18 Months of Age: Exploring Changes in Growth and Skinfold Thicknesses. Pediatr Res 70, 368–374 (2011). https://doi.org/10.1203/PDR.0b013e318229007b
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318229007b
This article is cited by
-
Developmental origins of NAFLD: a womb with a clue
Nature Reviews Gastroenterology & Hepatology (2017)
-
Effects on metabolic markers are modified by PPARG2 and COX2 polymorphisms in infants randomized to fish oil
Genes & Nutrition (2014)
-
Effect of dietary intervention to reduce the n-6/n-3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age
European Journal of Clinical Nutrition (2013)