Abstract
The fecal microbiota of 37 infants with (n = 20) or without (n = 17) probiotic administration was evaluated on D 3, and at 1, 3, and 12 mo by fluorescence in situ hybridization-flow cytometry (FISH-FC), PCR, and bacteriological culture methods. They represent consecutive subjects of an ongoing double-blind, placebo-controlled trial on a probiotic formula (LGG and Bifidobacterium longum) administered during the first 6 mo of life. Despite varying composition in each baby, there was a general bacterial colonization pattern in the first year. Bifidobacteria increased markedly (p = 0.0003) with a parallel decrease in Enterobacteriaceae (p < 0.001) and Bacteroides–Prevotella (p = 0.005) populations. Eubacterium rectale–Clostridium coccoides (p < 0.001) and Atopobium (p = 0.039) groups also gradually increased. This overall pattern was unaffected by probiotic administration (p > 0.05). B. longum (p = 0.005) and Lactobacillus rhamnosus (p < 0.001) were detected more frequently in probiotic group during supplementation, but no difference after supplementation had ceased (p > 0.05). Cultured lactic acid bacteria were also more numerous in the probiotic-administered babies during treatment period (log10 CFU/g 8.4 versus 7.4; p = 0.035). Our results indicate that supplemented strains could be detected but did not persist in the bowel once probiotic administration had ceased.
Similar content being viewed by others
Main
The developmental origins of health and disease” hypothesis proposes that the risk of developing chronic diseases can be explained, at least in part, by the influence of environmental cues acting very early in life (1,2). The acquisition of the intestinal microbiota may be a key event because, in experimental animal models, the presence of a microbiota is associated with alterations to host physiology (3,4). The dynamics of the colonization of the infant intestine is still largely unexplored because studies based on culture methods reveal only a small fraction of the total bacterial community (5–7). Molecular biologic tools now provide the means to investigate the topic in depth (8,9).
The hygiene hypothesis states that lack of exposure to pathogens or certain commensal bacteria in early life may predispose some individuals toward manifestation of allergic disorders (10). The gut microbiota plays an important role in the regulation of immune deviation and an imbalance in its composition may lead to an increased susceptibility toward allergies (11). The use of probiotic bacteria as prophylactic agents from birth may, however, serve as a means to reverse the imbalance (12). Probiotics are “live microorganisms, which when administered in adequate amounts confer a health benefit on the host” (13). It is uncertain, however, whether probiotic supplementation of the diet can alter the overall pattern in which bacteria colonize the infant gut. Most studies have concentrated on adult subjects and reported a transient passage of probiotic bacterial cells through the gut during the period of administration (14–17).
We have followed the establishment of the gut microbiota in Asian newborns during the first year of life using both bacterial culture-based and nucleic acid-based techniques. Our aim was to determine whether the dynamics of bacterial colonization of the infant gut would be altered by administration, during the first 6 mo of life, of a probiotic product containing bifidobacteria and lactobacilli.
MATERIALS AND METHODS
Subject recruitment and study design.
The subjects were a subset of consecutively recruited infants participating in an on-going clinical trial conducted at the National University Hospital, Singapore. To minimize selection bias, consecutive subjects (subject codes PR048 to PR088) were recruited to form this sub-cohort as stool collection for these studies commenced from subject 48. Subjects were recruited between 4 February 2005 and 9 June 2005. Expectant mothers carrying infants at risk of developing allergic disease (at least one first-degree relative with a history of allergic disease) were invited to participate in this randomized, double-blind, placebo-controlled trial on the use of a probiotic bacteria-supplemented cow's milk-based infant formula in the first six months after birth. The inclusion criteria were 1) first-degree relative with a history of allergic disorder as confirmed by a doctor's diagnosis of asthma, allergic rhinitis, or eczema and a positive skin prick test to any of a panel of common dust mite allergens, which are the most important inhalant allergens in our atopic population (18); 2) gestational age above 35 wk and birth weight above 2 kg; 3) absence of major congenital malformations or major illness at birth; 4) deemed to be in good health based on medical history and physical examination; and 5) the family assessed to be able to complete the trial. Mothers who intended to fully breast feed their infants were not considered for this study. In a double-blinded manner, newborns were randomized into blocks of six subjects to receive either infant formula supplemented with probiotic bacteria [Bifidobacterium longum BB536 and Lactobacillus rhamnosus GG (LGG)] or standard formula as control for the first six months. Randomization and labeling was carried out by the infant formula manufacturers (Nestle, Vevey, Switzerland). Both kinds of formula feed (test and control) tasted and appeared identical. Written, informed consent was obtained from all families. The study was approved by the National University Hospital's ethics review committee (Ref Code: B/00/322).
Formula feeds.
Commercial infant formula feeds (Nan 1) with or without probiotic bacteria, were provided by Nestle. Both probiotic supplemented and control formula was not hydrolyzed and not supplemented with prebiotics. Quality control testing by the manufacturer showed that the probiotic bacteria in the formulation remained viable during 600 d. The probiotic group received infant formula containing 1 × 107 colony forming units per gram (CFU/g) of B. longum BB536, and 2 × 107 CFU/g of L. rhamnosus GG. The infants ingested a minimum of 60 mL of formula daily so that the infants in the probiotic group received at least 109 CFU of probiotic bacteria per day, which was deemed sufficient according to a previous study (19). Mothers were then free to decide whether to make up the remainder of the baby feeds with either the trial formula, or to supplement with breast milk or another infant formula. The control group received the same formula without the probiotic bacteria. At the time of the trial, commercial infant formulas (first 6 mo) containing probiotic bacteria were not available in Singapore, hence eliminating the possibility of babies receiving probiotic bacteria from other sources. Compliance to the taking of at least 60 mL of formula was ensured by filling of diary records by caregivers, and phone and clinic contact with subjects and caregivers, as well as a record on the usage of test formula.
Weaning to solids was allowed from 4 to 6 mo of age, according to local practices. Parents were advised to avoid highly allergenic weaning foods such as eggs, peanuts and shellfish.
Sample collection and preparation.
Fecal samples were collected on d 3, and at 1, 3, and 12 mo after birth into sterile plastic vials by parents, stored at –20°C and delivered to the laboratory within 20 h. The samples were kept cool on a dry-ice pack during transport and, immediately upon arrival at the laboratory, diluted with 0.85% sodium chloride solution (saline) to give a 0.1 g/mL homogenate. After preparation of the homogenate, samples were used for bacteriological culture, fixation in 4% paraformaldehyde, and storage in TN (10 mM Tris-HCl [pH 8], 150 mM NaCl) buffer for later DNA extraction.
Fluorescence in situ hybridization combined with flow cytometry (FISH-FC).
A panel of seven nonoverlapping bacterial phylogenetic probes was used (Table 1). They covered the major constituents of the human fecal microbiota as determined in adult samples. Fixation of fecal samples in 4% paraformaldehyde and FISH-FC were performed as described previously by Lay et al. (9). As fecal samples were homogenized in a volume of saline according to weight regardless of consistency, a universal probe EUB338 was used as positive control (Table 1) and each phylotype was expressed as percentage of total EUB338 to provide standardization to the FISH-FC assay.
DNA extraction. Bacterial DNA from pure cultures was extracted using the method described previously by Knarreborg et al. (19). Aliquots of fecal homogenates (0.1 g/mL) were centrifuged at 14,800 g for 5 min, subsequently washed and resuspended in 500 μL of TN buffer. The suspension was transferred into a 2 mL Eppendorf tube containing 0.3 g of 0.1 mm Zirconia/silica beads (Biospec, Inc., Bartlesville, OK) and was homogenized at maximum speed for 3 min with a mini bead-beater (Biospec, Inc.). The sample was then transferred to ice for 5 min. The tube was centrifuged at 14,800 g for 15 min and 500 μL of the supernatant was transferred to a new tube for DNA extraction. The aqueous supernatant containing DNA was subsequently subjected to two phenol-chloroform (1:2) extractions. The DNA was precipitated with 1 mL of ethanol and 50 μL of 3 M sodium acetate. The sample was vortexed and left overnight at –20°C. After centrifugation for 15 min at 14,800 g (4°C), the DNA pellet was air-dried at 37°C for approximately 1.5 h. The DNA was dissolved in 25 μL of sterile TE buffer (pH 8.0), and was then ready for PCR.
PCR.
Specific PCR primers targeting bifidobacterial species and L. rhamnosus GG (LGG), as well as their respective PCR conditions used, are listed in Table 2. The bifidobacterial primers produced amplicons of 301, 278, 827, 1197, and 828 bp representing B. longum, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium adolescentis, and Bifidobacterium infantis, respectively. These primers sets were previously reported by Kwon et al. (21) and Matsuki et al. (22). LGG-specific PCR targeting a phage-related sequence found in the genome of LGG produced an amplicon of 470 bp, as described by Brandt and Alatossava (23).
Detection of various bacterial species in fecal DNA was made using a single PCR machine (GeneAmp PCR system 9700, Applied Biosystems, Foster City, CA). The size of PCR products was checked on a 2% agarose gel (Seakem, Rockland, ME) containing ethidium bromide (5 μg/mL).
Culture.
As described previously (24), colony counts and identification of the various bacterial types were based on growth, color, and number of colonies on selective media, Gram staining and cell morphology. Selective media used were de Man-Rogosa-Sharpe agar (MRS) (Oxoid) for lactic acid bacteria (LAB), Slanetz and Bartley agar (S&B) (Oxoid Ltd., Basingstoke, UK) for Enterococcus spp., MacConkey agar (Oxoid Ltd.) for coliforms and Staphylococcus medium no. 110 (Oxoid Ltd.) for Staphylococcus spp. The samples were serially diluted (10–1–10–9) in saline in a biosafety cabinet. The MRS agar plates were incubated at 37°C, 5% CO2 for 48 h, MacConkey purple agar plates at 35°C aerobically, overnight, S&B agar plates at 35°C for 2 h and then at 44°C for 44 h, and Staphylococcus no. 100 agar at 35°C aerobically for 48 h. Bacteria were quantified employing a drop-plate technique by spotting 25 μL of each dilution on the same plate (24).
Statistics.
All statistical analyses were carried out using SAS version 9.1 (SAS Institute Inc., Cary, NC). Bacterial plate counts were expressed as log10 CFU/g of wet fecal samples and the lower detection limit was 3.6/g. The bacterial counts of probiotic and control groups after the log10 transformation were compared using two-samples t test whereas the occurrence of PCR amplicons was assessed by simple logistic regression analysis at each time point. Mixed effect model accounted for breast feeding status for the first 3 mo (not breast fed versus any breast feeding) was used to compare the bacterial counts and numerical succession of broad phylogenetic bacterial groups of probiotic and control groups, as well as the trends of bacterial counts and numerical succession over the first 3 mo after birth. This method allows for specification of the variance-covariance matrix structure to describe the relation between the correlated longitudinal measurements. The time from birth (in months) was considered as a random effect in the model. Similarly, generalized linear model adjusted for breast-feeding status was used to assess the prevalence of various PCR amplicons between the two groups and trends over time. The statistical significant was set at p < 0.05.
Based on a study by Gueimonde et al. (25), who reported a higher occurrence of L. rhamnosus at 6 mo of age in a probiotic group (78%) compared with controls (43%), we estimated a sample size of 26 subjects per group as adequate to detect a difference of 35% for a one-sided test of 5% and a power of 80%. As the addition of LGG into food and health supplements is common in Finland, but not Singapore, we expect a lower prevalence of this strain in our local cohort. Assuming LGG would be detected in 21.5% of our control infants, 12 subjects per group is deemed sufficient.
RESULTS
Clinical characteristics.
As the clinical aspects of the study were still blinded at the time of bacteriological analysis, we describe here the results of a subset of the cohort who were unblinded and analyzed by an independent statistician in relation to their bacteriological parameters. Forty-one consecutive subjects who completed 12 mo follow up were recruited to this study but within this group, there were four withdrawals, two subjects from each group. Data were analyzed from 20 and 17 infants from the probiotic and control group, respectively. The probiotic and control groups were similar in distribution for factors that are possible confounders, including birth weight, mode of delivery, and breast-feeding status. None of the infants received antibiotics during the neonatal period (Table 3). At the 1-y follow up, majority of them (28/37) were sensitized to dust mites, Dermatophagoides pteronyssinus and/or Blomia tropicalis, and 7 were diagnosed with eczema.
FISH-FC.
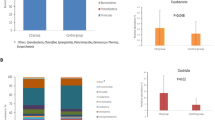
The proportions of the major phylogenetic bacterial groups within the total microbiota are given in Table 4, and did not differ at any of the sampling times between probiotic and control groups. There was an expansion in the size of the bifidobacterial population (p < 0.001) during the first 3 mo of life. Likewise, the E. rectale–C. coccoides (p = 0.034) and Atopobium groups (p = 0.007) also gradually increased over the first year. In contrast, Enterobacteriaceae (p < 0.001) and the Bacteroides-Prevotella group (p = 0.543) tended to diminish over time. Members of the C. leptum subgroup were rarely detected in the first 3 mo, and formed only a small fraction of the total fecal microbiota at 1 y of age. An area graph (Fig. 1) is provided to clearly display the general bacterial colonization pattern.
Colonization pattern over time of seven predominant bacterial phylotypes in infants administered with (A) probiotic bacteria (n = 19) or (B) control (n = 17) as determined by FISH-FC. Mean values of the two groups are shown. Bifidobacterium (BIF), Enterobacteriaceae (ENTER), Bacteroides-Prevotella (BAC), Atopobium (ATO), E. rectale–C. coccoides (EREC), Lactobacilli-Enterococci (LAB), Clostridium leptum (CLEP). GAP is defined as the bacterial proportion not detected by the selected panel of oligonucleotide probes.
Culture-based results.
After adjusting for breast-feeding status, the counts of LAB (p = 0.009), but not coliforms (p = 0.657), enterococci (p = 0.954), and staphylococci (p = 0.372), of infants in the probiotic group were significantly higher than those of the control group at 3 mo of life (Fig. 2). Counts of LAB (p = 0.002), coliforms (p < 0.001), and staphylococci (p < 0.001) decreased with age in both groups, whereas enterococcal counts showed an increase (p = 0.003).
PCR detection.
Table 5 summarizes the prevalence of L. rhamnosus and bifidobacterial species in fecal samples. L. rhamnosus was detected more commonly in the feces of infants who received the probiotic product (p < 0.001). Cessation of probiotic administration resulted in similar detection levels of this microorganism in the two infant groups at 12 mo (45% probiotic, 47% control, p = 0.900). B. longum was also detected more frequently in the probiotic group at 3 d of age (p = 0.009), but this difference was not significant at subsequent time points. The prevalence of B. longum in the feces increased 52% at 12 mo from ages of 3 d. After adjusting for the breast-feeding status, significantly more L. rhamnosus and B. longum were observed in the probiotic group over their first 3 mo of life [L. rhamnosus (odds ratio 111.93, 95% confidence interval: 23.18–540.45, p < 0.001)], B. longum (OR 3.75, 95%CI: 1.27–11.07, p = 0.017), and both L. rhamnosus and B. longum (OR 28.77, 95%CI: 6.48–127.68, p < 0.001). On the other hand, no statistical difference in the prevalence of other bifidobacterial species was observed between probiotic and control groups. From 3 d to 12 mo of age, the prevalence of B. breve increased dramatically (41% in control and 45% in probiotic), whereas B. bifidum detection rates were constant. B. infantis and B. adolescentis were rarely detected.
DISCUSSION
In general, a mixture of Gram-positive and Gram-negative bacteria dominated the fecal microbiota during the first month of life, whereas Gram-positive bacteria predominated by 12 mo of age. Bifidobacteria, enterobacteria, and Bacteroides-Prevotella were predominant members of the fecal microbiota during the first month of life. Thereafter, populations of obligately anaerobic phylotypes expanded, most notably the E. rectale–C. coccoides group, whereas enterobacterial and Bacteroides-Prevotella populations decreased as a proportion of the total microbiota. Members of the C. leptum subgroup were rarely detected in the feces of children less than 3 mo of age. On reaching 12 mo of age, atopobial and C. leptum populations were also more apparent and would presumably continue to expand until they comprise major proportions of the adult microbiota (8,26). In contrast, bifidobacteria, which were the predominant group (>47%) in the infants, account for only a few percent of the microbiota of adults (8). In summary, a distinctive succession of bacterial phylotypes occurred in the bowel of Asian infants at risk of atopic diseases even though the composition of individual microbiota was idiosyncratic. This general colonization pattern was not influenced by probiotic administration. The panel of oligonucleotide probes used in our study detected between 70% and 96% of the bacteria comprising the fecal microbiota of infants. The remaining fraction of the microbiota, known as the “phylogenetic gap,” was smaller than that reported for adults (8), showing a more limited biodiversity in infants.
In a recent allergy trial on high-risk infants, Kukkonen et al. (27) reported a higher cultured numbers of the supplemented strains during period of probiotic administration, but not 18 mo after supplementation had ceased. The detection of probiotic strains in feces using molecular methods has also been reported for both adults and neonates (1,14–17,28). We also detected probiotic bacteria, at least their DNA sequences, during the period of dietary supplementation with the probiotic product. Furthermore, we recorded higher lactic acid bacteria counts in the treatment group during probiotic administration compared with the control infants. There was an absence of evidence indicating permanent establishment (colonization) by the probiotic bacteria. This was readily observed with the combined data for L. rhamnosus and B. longum, where detection was not different between test and control groups at 12 mo of age (6 mo after probiotic administration had ceased). The “colonization resistance” concept (niche exclusion principle) predicts that an established microbial community prevents the establishment of further bacteria that need to occupy ecological niches that have already been filled. Thus, probiotic bacteria, which are allochthonous to the bowel ecosystem, would be unlikely to establish in the gut where a bacterial community has already been established (29). In support of this proposition, the results of our study show that probiotic administration does not result in alterations to the general colonization pattern during the first year of life. Although the two probiotic strains used in our study were originally isolated from humans, they are still allochthonous to other individuals because of the huge variation in bowel microbiota composition of humans (30). Furthermore, since the gut is sterile in utero, probiotic administration from birth could be the best strategy to enable establishment of probiotic bacteria. Our findings on both L. rhamnosus and B. longum show, however, that probiotic administration even from birth does not result in higher prevalence of the probiotic bacteria in the feces beyond the period of dietary supplementation (25). Unknown physiologic factors must dictate which bacteria, and in what proportions, colonize the bowel of individual humans (31).
Although the PCR primers used to detect L. rhamnosus DNA have been reported to be strain-specific, amplicons were obtained from the feces of a proportion of the control group. The addition of LGG to food or dietary supplements is uncommon in Singapore and it is highly unlikely that the control infants were exposed to this strain. Therefore, the strain specificity of the primers must be questioned and it seems likely that L. rhamnosus strains other than LGG were detected in the feces of the control group. Nevertheless, L. rhamnosus DNA was detected with a higher prevalence in the feces of probiotic-treated subjects than in fecal samples from control infants, indicating that we were able to detect the presence of the probiotic strain upon a background of naturally occurring strains of this bacterial species.
In conclusion, probiotic bacterial strains were only detected in the feces of the treated group during the period of administration and hence have a transient existence in the bowel as shown in studies with adult humans (30). The 2-y clinical follow-up of the infants in our study is expected to be completed by mid-2008, and will reveal whether the transit of B. longum and L. rhamnosus impacted on the prevalence of atopic disease. The data that we present here provide an essential bacteriological basis to understand the clinical outcomes of probiotic administration in early life. The potential to associate individual microbiota signatures with cases of atopy will be of particular interest in the future.
Abbreviations
- LAB:
-
lactic acid bacteria
References
Gillman MW 2005 Developmental origins of health and disease. N Engl J Med 353: 1848–1850
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE 2006 Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118: 511–521
Hooper LV, Gordon JI 2001 Commensal host-bacterial relationships in the gut. Science 292: 1115–1118
Tannock GW 2005 Commentary: remembrance of microbes past. Int J Epidemiol 34: 13–15
Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, Wilkinson MH, Welling GW 1995 Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol 61: 3069–3075
Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Dore J 1999 Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol 65: 4799–4807
Wilson KH, Blitchington RB 1996 Human colonic biota studied by ribosomal DNA sequence analysis. Appl Environ Microbiol 62: 2273–2278
Lay C, Rigottier-Gois L, Holmstrom K, Rajilic M, Vaughan EE, de Vos WM, Collins MD, Thiel R, Namsolleck P, Blaut M, Dore J 2005 Colonic microbiota signatures across five northern European countries. Appl Environ Microbiol 71: 4153–4155
Lay C, Sutren M, Rochet V, Saunier K, Dore J, Rigottier-Gois L 2005 Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ Microbiol 7: 933–946
Strachan DP 2000 Family size, infection and atopy: the first decade of the “hygiene hypothesis.”. Thorax 55: S2–S10
Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M 2001 Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 108: 516–520
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E 2001 Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357: 1076–1079
Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, Roberfroid M, Rowland I, Cherbut C, Klaenhammer TR 2003 New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37: 105–118
Garrido D, Suau A, Pochart P, Cruchet S, Gotteland M 2005 Modulation of the fecal microbiota by the intake of a Lactobacillus johnsonii La1-containing product in human volunteers. FEMS Microbiol Lett 248: 249–256
Rochet V, Rigottier-Gois L, Sutren M, Krementscki MN, Andrieux C, Furet JP, Tailliez P, Levenez F, Mogenet A, Bresson JL, Meance S, Cayuela C, Leplingard A, Dore J 2006 Effects of orally administered Lactobacillus casei DN-114 001 on the composition or activities of the dominant faecal microbiota in healthy humans. Br J Nutr 95: 421–429
Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP 2001 Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67: 2578–2585
Yamano T, Iino H, Takada M, Blum S, Rochat F, Fukushima Y 2006 Improvement of the human intestinal flora by ingestion of the probiotic strain Lactobacillus johnsonii La1. Br J Nutr 95: 303–312
Chew FT, Lim SH, Goh DY, Lee BW 1999 Sensitization to local dust-mite fauna in Singapore. Allergy 54: 1150–1159
Knarreborg A, Simon MA, Engberg RM, Jensen BB, Tannock GW 2002 Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microbiol 68: 5918–5924
Langhendries JP, Detry J, Van Hees J, Lamboray JM, Darimont J, Mozin MJ, Secretin MC, Senterre J 1995 Effect of a fermented infant formula containing viable bifidobacteria on the fecal flora composition and pH of healthy full-term infants. J Pediatr Gastroenterol Nutr 21: 177–181
Kwon HS, Yang EH, Lee SH, Yeon SW, Kang BH, Kim TY 2005 Rapid identification of potentially probiotic Bifidobacterium species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol Lett 250: 55–62
Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H 1999 Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl Environ Microbiol 65: 4506–4512
Brandt K, Alatossava T 2003 Specific identification of certain probiotic Lactobacillus rhamnosus strains with PCR primers based on phage-related sequences. Int J Food Microbiol 84: 189–196
Mah KW, Bjorksten B, Lee BW, van Bever HP, Shek LP, Tan TN, Lee YK, Chua KY 2006 Distinct pattern of commensal gut microbiota in toddlers with eczema. Int Arch Allergy Immunol 140: 157–163
Gueimonde M, Kalliomaki M, Isolauri E, Salminen S 2006 Probiotic intervention in neonates—will permanent colonization ensue?. J Pediatr Gastroenterol Nutr 42: 604–606
Hayashi H, Sakamoto M, Kitahara M, Benno Y 2006 Diversity of the Clostridium coccoides group in human fecal microbiota as determined by 16S rRNA gene library. FEMS Microbiol Lett 257: 202–207
Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M 2007 Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 119: 192–198
Marzotto M, Maffeis C, Paternoster T, Ferrario R, Rizzotti L, Pellegrino M, Dellaglio F, Torriani S 2006 Lactobacillus paracasei A survives gastrointestinal passage and affects the fecal microbiota of healthy infants. Res Microbiol 157: 857–866
Atlas RM 1999 Probiotics—snake oil for the new millennium?. Environ Microbiol 1: 377–382
Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart Gopal JK 2000 Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol 66: 2578–2588
Rawls JF, Mahowald MA, Ley RE, Gordon JI 2006 Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127: 423–433
Acknowledgements
The authors thank Karen Munro and Or Ming Yan for technical assistance, and Dr Yvonne Ng Peng, Judy Anthony, Corinne Kwek Poh Lian, and Soh Shu E for their excellent work in the clinic. We also thank Nestle for providing us the infant formula with and without probiotic bacteria, and preparing the randomization list. The voluntary participation of all subjects in the study is sincerely appreciated.
Author information
Authors and Affiliations
Additional information
The study was supported by National Medical Research Council (NMRC0971/2005), Republic of Singapore.
Rights and permissions
About this article
Cite this article
Mah, K., Chin, V., Wong, W. et al. Effect of a Milk Formula Containing Probiotics on the Fecal Microbiota of Asian Infants at Risk of Atopic Diseases. Pediatr Res 62, 674–679 (2007). https://doi.org/10.1203/PDR.0b013e31815991d5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31815991d5
This article is cited by
-
Compositional Quality and Possible Gastrointestinal Performance of Marketed Probiotic Supplements
Probiotics and Antimicrobial Proteins (2022)
-
The Role of Probiotics in the Prevention and Treatment of Atopic Dermatitis in Children: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials
Pediatric Drugs (2020)
-
Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: a randomized controlled trial
Pediatric Research (2018)
-
Molecular analysis of infant fecal microbiota in an Asian at-risk cohort–correlates with infant and childhood eczema
BMC Research Notes (2014)
-
Association between funding source, methodological quality and research outcomes in randomized controlled trials of synbiotics, probiotics and prebiotics added to infant formula: A Systematic Review
BMC Medical Research Methodology (2013)