Abstract
Twenty term/near term neonates with hypoxemic respiratory failure and oxygenation index ≥20 were enrolled in a Phase I/II feasibility, safety and dose escalation study of inhaled PGE1 (IPGE1). Incremental doses of IPGE1, delivered by a jet nebulizer over a 2-h period, followed by weaning over 1 h, were given to 13 patients before receiving inhaled nitric oxide (INO) (Group I), and to seven patients, who failed to respond to INO (Group II). Response was defined as an increase in PaO2 of either ≥ 25 (full) or 10–25 (partial) torr. Exit criteria included an acute deterioration in oxygenation status, a persistent oxygenation index above 35 in Group I, or the availability of extracorporeal membrane oxygenation (ECMO) in Group II. The mean (SD) increase in PaO2 at the end of IPGE1 administration was 63 (62.3) in Group I (p = 0.024), and 40 (62.1) in Group II (p > 0.05). In Group I, 8 of 13 neonates had a full response, but 4 deteriorated following discontinuation of IPGE1. Of these four, two responded to INO and two were placed on ECMO. Five patients deteriorated before or during IPGE1, and none of them responded to INO. In Group II, three of seven patients had a full response to IPGE1. One patient with a partial response and all patients exiting before or during IPGE1 administration were placed on ECMO. The results of our study indicate that IPGE1 may be a safe, selective pulmonary vasodilator in neonatal hypoxemic respiratory failure.
Similar content being viewed by others
Main
Hypoxemic respiratory failure in the newborn is usually associated with potentially reversible pulmonary hypertension that causes right-to-left shunting and profound hypoxemia. The goal of therapy is to selectively lower the pulmonary vascular resistance (PVR). Intravenously administered vasodilators lack pulmonary selectivity leading to systemic side effects. Inhaled nitric oxide (INO), a selective pulmonary vasodilator, has revolutionized the treatment of hypoxemic respiratory failure. However, there is lack of sustained improvement in 30–46% of infants (1–4); moreover, INO is a highly toxic molecule requiring expensive monitoring and scavenging systems for administration, making the treatment expensive and limiting availability. Aerosolized prostaglandins I2 and E1 have been reported to be effective selective pulmonary vasodilators in animals and human adults (5–19). In addition, inhaled PGI2 (IPGI2) has also been reported to be effective in preterm and term newborns and children with pulmonary hypertension (20–26). Although i.v. PGE1 is widely used in neonates, the use of the inhaled form has not been reported in newborns with pulmonary hypertension. The high pulmonary clearance of PGE1 (70 to 90%) contributes to its selectivity as a pulmonary vasodilator when administered as an aerosol (17). Compared with PGI2, PGE1 has a shorter half-life, lower pKa (6.3 versus 10.5), bronchodilator action, anti-proliferative and anti-inflammatory effects on the alveolar, and interstitial and vascular spaces of the lung (17,27–31). Prostaglandin nebulization can be used without the sophisticated technical equipment needed for controlled NO inhalation and hence is less expensive. It has no known toxic metabolites or toxic effects. Prostaglandins and nitric oxide relax the vascular smooth muscles through two different second-messenger systems; therefore, in combination, INO and IPGE1 may have synergistic effect (32).
The existing literature suggests that inhaled PGE1 is a potential effective vasodilator in the treatment of pulmonary hypertension of the newborn. We report the results of a phase I-II open-label pilot study of escalating doses of aerosolized PGE1 in term/near-term neonates with hypoxemic respiratory failure. Our objectives were to establish the feasibility and safety of PGE1 administered as an aerosol and to determine the effective dose.
METHODS
Subjects.
The study was conducted at five centers in the metropolitan Detroit area. The IRB at all five hospitals approved the study. All of the patients in this report were recruited at two of the five centers (Children's Hospital of Michigan and Hutzel Women's Hospital). INO was available only during transport to and at Children's Hospital of Michigan. Newborn infants born at ≥34 wk gestation requiring assisted ventilation for hypoxemic respiratory failure in the first 2 weeks of life were eligible to participate after an oxygenation index (OI) of ≥ 20 on two arterial blood gases at least 15 min apart in the preceding 12 h. Additional requirements were an indwelling arterial catheter and informed parental consent. Although an attempt was made to obtain a head ultrasound and cardiac echocardiogram before enrollment, this was not a requirement for study participation. Neonates with congenital diaphragmatic hernia, congenital heart disease other than ductal or septal shunts, thrombocytopenia, or those in whom a decision to not provide full treatment (including ECMO) were considered ineligible for the study.
Two groups of patients were defined based on disease severity and prior treatment with INO at the time of enrollment. Patients in Group I (Pre-INO Group) were enrolled before they received INO. Group II patients (Post-INO Group) were enrolled after they were found to be refractory to INO. A patient was labeled as being “refractory to INO” if there was no response to INO one hour after initiation or if there was a failure to sustain a response in PaO2 ≥25 mm Hg above baseline at any time without weaning of INO or ventilator. Many of the patients in Group II were eligible and were waiting for the availability of ECMO.
Management of eligible infants was optimized before treatment with aerosolized PGE1 by the clinical team. This included management decisions about the use of conventional or high frequency oscillatory ventilation, induction of alkalosis, administration of volume, surfactant, pressors, sedation and paralysis. Surfactant therapy was initiated before the initiation of IPGE1. The mode of ventilation remained unchanged for the duration of the study.
Drug dosing and administration of aerosolized PGE1.
PGE1 solution for aerosolization was prepared from 500 μg synthetic PGE1 dissolved in 1 mL ethanol (Gensia Sicor Pharmaceuticals, Irvine, CA) by dilution in 0.9% saline. Once diluted, the solution was used within 24 h. Four different doses of aerosolized PGE1 (25, 50, 150, and 300 ng/kg/min) were prepared. These trial doses were selected based on the recommended i.v. dose of 50 to 100 ng/kg/min (0.05 to 0.1 μg/kg/min) for ductal patency in neonates with congenital cardiac lesions. The dose was varied by a factor of three from the recommended i.v. dose of 100 ng/kg/min. The PGE1 concentration in the solution was varied to keep the nebulized volume constant at 2.2 to 2.6 mL/h. Inhalation was begun at the lowest dose and each dose was administered for 30 min in all infants. Once the maximal dose was achieved (300 ng/kg/min), IPGE1 was weaned in 15 min steps (weaning phase). The entire study lasted for 3 h unless the infant met exit criteria before completion.
The PGE1 dosage refers to the total amount of nebulized drug placed in the nebulizer. It has previously been shown that the fraction deposited in the alveolar space during mechanical ventilation is less than 10 to 20% of the dose administered (10). The alveolar dose is even smaller in newborn infants as there is proportionally larger dead space (22).
Aerosols of PGE1 with a mean particle size of 2 to 3 μm were generated with a jet nebulizer (miniHeart, Westmed Inc., Lakewood, CO). The nebulizer was connected to the inspiratory limb of the ventilator (Fig. 1). During the inhalation period, the ventilator flow was adapted according to the additional flow of the nebulizer to maintain alveolar ventilation and inspired oxygen concentration. Baseline conditions were assessed by aerosolization of normal saline (2 mL/h) for 15 min before the first dose of aerosolized PGE1 (25 ng/kg/min).
Monitoring during administration of aerosolized PGE1.
Hemodynamic parameters and gas exchange (arterial blood gas) were assessed before PGE1 administration, after each dose change and 60 min after withdrawal of the final dose. Blood counts, serum electrolytes and liver enzymes were obtained before and 24 h after concluding the study. The infants were monitored clinically for hyperthermia, hypotension, dysrhythmias, signs of bleeding, and seizures.
Outcome variables.
The primary outcome variable was the change in PaO2 from baseline after 30 min exposure to aerosolized PGE1 and at end of IPGE1 administration. To determine this, an arterial blood gas analysis was performed after 30 min of exposure to each dose of IPGE1. A response was defined as an increase in PaO2 ≥ 25 (full), 10–25 (partial) or <10 (none) mm Hg. Secondary outcome variables included change in oxygenation index, need for nitric oxide and/or ECMO, mortality, and duration of mechanical ventilation, oxygen therapy, and hospitalization.
Exit Criteria.
Infants in both groups exited the study if there was an acute drop in pulse oximeter saturation of >10% for which no mechanical cause could be identified. Additionally, infants in Group I exited the study if the OI exceeded 35 for more than 30 min during the study in the absence of mechanical cause for the worsening oxygenation. Infants in Group II awaiting ECMO, exited when ECMO was available.
Statistical Analyses.
We estimated a sample size of 17 to determine minimum difference in PaO2 before and after IPGE1 of 25 mm Hg, a SD of 30 mm Hg, assuming a power of 90, and alpha of 0.05 (2-tailed). The sample was increased to 20 to account for infants exiting the study.
Statistical analysis was done using an intention-to-treat analysis. We evaluated categorical variables using χ2 tests. Continuous variables were compared with paired or independent samples t tests and ANOVA. Longitudinal data were analyzed using the mixed procedure in SAS. Significance level was set at 0.05.
RESULTS
Baseline Characteristics
Twenty infants were enrolled in the trial; 13 in Group I and 7 in Group II. The baseline maternal, perinatal, and postnatal characteristics are summarized (Table 1). Air leaks were present in three (15%), pulmonary hemorrhage in two (10%), and seizures in two infants (10%) before enrollment.
Cardio-respiratory variables at enrollment are summarized (Table 2). All infants in Group I had at least one OI ≥25, 10 had at least 2 such OIs. Although all infants had an echocardiogram at some point in the acute course of their illness, 85% (n = 17) had an echocardiogram before enrollment; of these, 82.4% had evidence of pulmonary hypertension (right-to-left or bidirectional shunting, systemic or supra-systemic right ventricular pressure or a diagnosis by the cardiologist of pulmonary hypertension).
Conventional therapy before enrollment included volume resuscitation (100%), sedation/analgesia (100%), vasopressors (95%), steroids for hypotension (40%), surfactant (60%), neuromuscular blockade (45%), and i.v. PGE1 (5%). Twenty-five percent of the infants had respiratory alkalosis (defined as pH >7.45 at enrollment). Ninety percent of the infants had a trial of high frequency oscillatory ventilation before enrollment; however, only 55% were on an oscillatory ventilator at the time of enrollment.
Primary Outcome: Change in PaO2 from Baseline
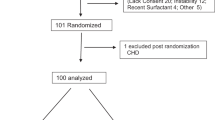
Fig. 2 is the flow diagram of enrolled subjects whose clinical descriptions are shown in Table 3.
Group I.
Two infants in Group I met exit criteria before receiving IPGE1. Three infants met exit criteria during the study. Eight infants completed the study and had a full response to IPGE1. At the end of the study, there was a significant increase in PaO2 (63.0 ± 62.3, p = 0.024), decrease in OI (13.0 ± 8.8, p = 0.004) and decrease in alveolar-arterial oxygen gradient (58.3 ± 65.2, p = 0.039).
Group II.
All infants in this group had demonstrated a lack of response to INO. Five received INO during transport from birth hospital to the referral center. Duration of INO therapy before enrollment was 6.1 h (median) (range 1.5 to 91 h). One infant exited the study without receiving IPGE1 and two infants exited during the study as ECMO was available. Four infants in Group II completed the IPGE1 study. One infant improved before receiving IPGE1. This infant demonstrated an additional full response to IPGE1. The mean± SD increase in PaO2 (39.8 ± 62.1), decrease in OI (7.6 ± 13.4) and alveolar-arterial oxygen gradient (39.3 ± 66.6), was not statistically significant compared with baseline (p > 0.05 for all) in these four infants.
Dose Response Effect
Among the eight subjects completing the study in Group I, a full response was observed in 50% after a dose of 50 ng/kg/min, and in 87.5% after a dose of 150 ng/kg/min. One patient (12.5%) had a partial response in the escalation phase, but showed a complete response during the weaning phase (Fig. 3). At the end of the weaning phase, five infants sustained a full response.
The x axis represents the dose of IPGE1 in ng/kg/min. The data points represent oxygenation at the end of each dose. A and B represent oxygenation in two arterial blood gases immediately before enrollment. C represents oxygenation at the end of nebulized normal saline. D represents oxygenation 1 h after discontinuing IPGE1. The hatched vertical line demarcates the escalation and weaning phases. The individual lines represent the subject-specific PaO2 profiles over time. In Group I, there is significant increase in PaO2 with increasing IPGE1 dose in the escalation phase; the response is sustained in the weaning phase in the group as a whole. In Group II, there is no association between the IPGE1 dose and PaO2 response in either the escalation or weaning phase. The between subjects variability is more marked in Group II compared with Group I.
In the four patients in Group II completing the IPGE1 study, a full response was observed in 25% after a dose of 50 ng/kg/min, in 50% after a dose of 150 ng/kg/min, and in 75% after a dose of 300 ng/kg/min (Fig. 3). At the end of the weaning phase, the three infants with a full response sustained it.
Analysis using Mixed Models (SAS) (Fig. 3) revealed a significant association of IPGE1 dose and PaO2 during the escalation phase in Group I (β = 11.4, SE = 3.5, p = 0.009) but not during weaning. In Group II, no association was observed either in the escalation phase or during weaning. A more complex model adjusting for time on IPGE1 and IPGE1 dose revealed a significant effect of both dose (β = 7.1, SE = 2.4, p = 0.02) and time (β = 6.2, SE = 1.1, p < 0.0001) for Group I and only a significant effect of time (β = 2.7, SE = 1.3, p = 0.0461) but not dose (β = 6.5, SE = 19.8, p = 0.8) for Group II.
Secondary Outcomes
Need for INO or ECMO.
Nine of the 13 infants in Group I (69.2%) received INO and 6 (46.2%) eventually were placed on ECMO. All infants in Group I who did not respond to IPGE1 (n = 5) also failed to respond to INO and all qualified for ECMO. Fifty percent (n = 4) of the infants with full response to IPGE1 in Group I continued to improve without need for INO or ECMO. The median time from the discontinuation of IPGE1 to initiation of INO in the four patients who did not sustain a response was 8.2 h (range 0.3 to 30 h). Of these, two did not respond to INO and were placed on ECMO 13.5 and 75 h after conclusion of the IPGE1 study. Four of the seven infants in Group II (57.1%) were placed on ECMO.
Mortality.
Three infants in Group I (23.1%) and 1 infant in Group II (14.3%) died before hospital discharge or 120 d of life (Table 4). Causes of death included fulminant sepsis (n = 1) and pulmonary hypoplasia (n = 3).
Results of cranial imaging studies. A cranial ultrasound was obtained in all patients during the acute phase of the illness. It was obtained before enrollment in 9 of 13 (69.2%) infants in Group I and 6/7 (85.7%) infants in Group II. Abnormal findings included cerebral edema, ventriculomegaly, Grade I IVH, and periventricular echogenicity. Abnormal findings in the initial sonogram obtained within 24 h after the IPGE1 study (n = 5) included Grade I IVH. No new findings or progression was observed in patients who had a sonogram both before enrollment and after the IPGE1 study in the acute phase. Cranial imaging findings before discharge or death included hydrocephalus (n = 1), stable ventriculomegaly (n = 7), extra-axial fluid (n = 10), infarction (n = 1) and periventricular leukomalacia (n = 2).
Toxicity/adverse effects.
Inhaled PGE1 was not discontinued in any infant because of adverse effects. No episodes of exacerbation of hypotension, hyperthermia, dysrhythmias, or bleeding tendency were observed. Paired t tests did not reveal significant differences in temperature, systolic blood pressure or heart rate at baseline and end of IPGE1 administration in either group. Results of blood counts and serum chemistries obtained before and 24 h after study conclusion are presented in Table 4. The hematocrit, total leukocyte count and aspartate aminotransferase were lower after IPGE1 whereas blood urea nitrogen was higher; however all values were within normal limits and the differences were not clinically significant. Seizures and ventriculomegaly were documented in two infants before enrollment in study. No new onset seizures were documented in any of the patients within 1 wk after enrollment. Mild ventriculomegaly was documented in babies who had undergone ECMO and is probably not attributable to the use of IPGE1 in these infants.
DISCUSSION
The results of this phase I-II study suggest that IPGE1 is a safe selective pulmonary vasodilator in hypoxemic respiratory failure at the concentrations and durations used in the trial. No evidence of tolerance, rebound pulmonary hypertension between doses and after cessation of IPGE1 or systemic side effects was detected.
Although a few studies have reported on the use of aerosolized PGI2 in newborns with hypoxemic respiratory failure, there are no reports on the use of IPGE1 in this population. De Jaegere et al. (20) reported improved oxygenation without adverse systemic effects in 4 preterm infants following endotracheal instillation of PGI2. Similar benefits were reported by Soditt et al. in a preterm newborn with pulmonary hypertension (21). Aerosolized PGI2 was associated with improvement in oxygenation in two term infants with persistent pulmonary hypertension and one infant with congenital heart disease (22,24). Sustained improvement in oxygenation was also reported by Kelly et al. in three of four term infants who had failed to respond to INO following administration of IPGI2 and milrinone (23). Milrinone has been shown to amplify the pulmonary vasodilatory response to inhaled PGI2 (33).
A clinically significant improvement in oxygenation was observed in the patients in Group I when IPGE1 was given for 3 h. This improvement was sustained during the weaning phase in this group. The magnitude of the improvement in oxygenation in this group is comparable to that reported previously for INO (1,2,34). In Group II, at least one infant had already received i.v. PGE1 at the referring hospital. This may have interfered with the selective pulmonary action of IPGE1. Three infants in this group did show a full response to IPGE1 and never qualified for ECMO. This provides credence to the speculation that INO and IPGE1 may have additive effects because of activation of different cellular mechanisms (32). The improvement in PaO2 was observed earlier and at lower IPGE1 dose in Group I patients compared with Group II patients. Previous studies have referred to the benefits of earlier institution of therapy for hypoxemic respiratory failure (1,35). The improvement in PaO2 was predicted by both dose and time in study for Group I but only by time in study for Group II. It is possible that a greater number of patients might have responded and sustained the response had the IPGE1 been administered for longer.
This study was designed to find a safe and effective dose of inhaled PGE1 and found that a dose of 150 to 300 ng/kg/min may be used safely and effectively in neonates with hypoxemic respiratory failure. The sample size is relatively small and heterogeneous without a placebo control group, not atypical for clinical phase 1 and phase 2 drug trials and can only detect large differences. The drug was administered for a short duration (3 h) and the efficacy and safety of a longer drug inhalation remains to be evaluated. This study describes for the first time the use of inhaled PGE1 in neonates with hypoxemic respiratory failure and suggests further placebo controlled randomized studies to establish efficacy and safety of this drug in this neonatal population.
Acknowledgments.
We gratefully acknowledge the assistance of Marilyn Dow, M.L.I.S., in searching the medical literature; Michael Barabash, B.S., R.R.T., in standardizing the nebulization technique; and Monica Malian, R.Ph., in developing a protocol for dosage of nebulized IPGE1. We also thank the parents who permitted their infants' participation in the study and the physicians, nurses, and members of the respiratory therapy staff at Children's Hospital and Hutzel Women's Hospital for support and assistance in performing our study.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- ECMO:
-
extracorporeal membrane oxygenation
- HUS:
-
head ultrasound
- INO:
-
inhaled nitric oxide
- IPCKD:
-
infantile polycystic kidney disease
- IPGE1:
-
inhaled Prostaglandin E1
- MRI:
-
magnetic resonance imaging
- OI:
-
oxygenation index
- PaO2:
-
arterial oxygen tension
- PG:
-
prostaglandin
- PGE1:
-
prostaglandin E1
- PGI2:
-
prostaglandin I2
- PPHN:
-
persistent pulmonary hypertension of the newborn
- PVL:
-
periventricular leukomalacia
References
The Neonatal Inhaled Nitric Oxide Study Group 1997 Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604
Roberts JD Jr, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM 1997 Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 336: 605–610
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP 2000 Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342: 469–474
Truog WE, Castor CA, Sheffield MJ 2003 Neonatal nitric oxide use: predictors of response and financial implications. J Perinatol 23: 128–132
Welte M, Zwissler B, Habazettl H, Messmer K 1993 PGI2 aerosol versus nitric oxide for selective pulmonary vasodilation in hypoxic pulmonary vasoconstriction. Eur Surg Res 25: 329–340
Zobel G, Dacar D, Rodl S, Friehs I 1995 Inhaled nitric oxide versus inhaled prostacyclin and intravenous versus inhaled prostacyclin in acute respiratory failure with pulmonary hypertension in piglets. Pediatr Res 38: 198–204
Zwissler B, Welte M, Messmer K 1995 Effects of inhaled prostacyclin as compared with inhaled nitric oxide on right ventricular performance in hypoxic pulmonary vasoconstriction. J Cardiothorac Vasc Anesth 9: 283–289
Booke M, Bradford DW, Hinder F, Harper D, Brauchle RW, Traber LD, Traber DL 1996 Effects of inhaled nitric oxide and nebulized prostacyclin on hypoxic pulmonary vasoconstriction in anesthetized sheep. Crit Care Med 24: 1841–1848
Olschewski H, Walmrath D, Schermuly R, Ghofrani A, Grimminger F, Seeger W 1996 Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med 124: 820–824
Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W 1996 Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med 153: 991–996
Webb SA, Stott S, van Heerden PV 1996 The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med 22: 353–355
Mikhail G, Gibbs J, Richardson M, Wright G, Khaghani A, Banner N, Yacoub M 1997 An evaluation of nebulized prostacyclin in patients with primary and secondary pulmonary hypertension. Eur Heart J 18: 1499–1504
Walmrath D, Schermuly R, Pilch J, Grimminger F, Seeger W 1997 Effects of inhaled versus intravenous vasodilators in experimental pulmonary hypertension. Eur Respir J 10: 1084–1092
Haraldsson A, Kieler-Jensen N, Nathorst-Westfelt U, Bergh CH, Ricksten SE 1998 Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest 114: 780–786
Max M, Kuhlen R, Dembinski R, Rossaint R 1999 Effect of aerosolized prostacyclin and inhaled nitric oxide on experimental hypoxic pulmonary hypertension. Intensive Care Med 25: 1147–1154
Krieg P, Wahlers T, Giess W, Rohde R, Hartrumpf M, Bund M, Haverich A 1998 Inhaled nitric oxide and inhaled prostaglandin E1: effect on left ventricular contractility when used for treatment of experimental pulmonary hypertension. Eur J Cardiothorac Surg 14: 494–502
Meyer J, Theilmeier G, Van Aken H, Bone HG, Busse H, Waurick R, Hinder F, Booke M 1998 Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg 86: 753–758
Putensen C, Hormann C, Kleinsasser A, Putensen-Himmer G 1998 Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157: 1743–1747
Lockinger A, Schutte H, Walmrath D, Seeger W, Grimminger F 2001 Protection against gas exchange abnormalities by pre-aerosolized PGE1, iloprost and nitroprusside in lung ischemia-reperfusion. Transplantation 71: 185–193
De Jaegere AP, van den Anker JN 1998 Endotracheal instillation of prostacyclin in preterm infants with persistent pulmonary hypertension. Eur Respir J 12: 932–934
Soditt V, Aring C, Groneck P 1997 Improvement of oxygenation induced by aerosolized prostacyclin in a preterm infant with persistent pulmonary hypertension of the newborn. Intensive Care Med 23: 1275–1278
Bindl L, Fahnenstich H, Peukert U 1994 Aerosolized prostacyclin for pulmonary hypertension in neonates. Arch Dis Child Fetal Neonatal Ed 71: F214–F216
Kelly LK, Porta NF, Goodman DM, Carroll CL, Steinhorn RH 2002 Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr 141: 830–832
Zwissler B, Rank N, Jaenicke U, Schurle B, Welte M, Reichart B, Netz H, Messmer K, Peter K 1995 Selective pulmonary vasodilation by inhaled prostacyclin in a newborn with congenital heart disease and cardiopulmonary bypass. Anesthesiology 82: 1512–1516
Santak B, Schreiber M, Kuen P, Lang D, Radermacher P 1995 Prostacyclin aerosol in an infant with pulmonary hypertension. Eur J Pediatr 154: 233–235
Pappert D, Busch T, Gerlach H, Lewandowski K, Radermacher P, Rossaint R 1995 Aerosolized prostacyclin versus inhaled nitric oxide in children with severe acute respiratory distress syndrome. Anesthesiology 82: 1507–1511
Cox JW, Andreadis NA, Bone RC, Maunder RJ, Pullen RH, Ursprung JJ, Vassar MJ 1988 Pulmonary extraction and pharmacokinetics of prostaglandin E1 during continuous intravenous infusion in patients with adult respiratory distress syndrome. Am Rev Respir Dis 137: 5–12
van Heerden PV, Caterina P, Filion P, Spagnolo DV, Gibbs NM 2000 Pulmonary toxicity of inhaled aerosolized prostacyclin therapy–an observational study. Anaesth Intensive Care 28: 161–166
Wasserman MA, Griffin RL, Marsalisi FB 1980 Inhibition of bronchoconstriction by aerosols of prostaglandins E1 and E2. J Pharmacol Exp Ther 214: 68–73
Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, Rennard SI, Crystal RG 1991 Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis 144: 1080–1084
Kato S, Sugimura H, Kishiro I, Machida M, Suzuki H, Kaneko N 2002 Suppressive effect of pulmonary hypertension and leukocyte activation by inhaled prostaglandin E1 in rats with monocrotaline-induced pulmonary hypertension. Exp Lung Res 28: 265–273
Lowson SM 2002 Inhaled alternatives to nitric oxide. Anesthesiology 96: 1504–1513
Haraldsson sA, Kieler-Jensen N, Ricksten SE 2001 The additive pulmonary vasodilatory effects of inhaled prostacyclin and inhaled milrinone in postcardiac surgical patients with pulmonary hypertension. Anesth Analg 93: 1439–1445
Day RW, Lynch JM, White KS, Ward RM 1996 Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 98: 698–705
Lonnqvist PA, Winberg P, Lundell B, Sellden H, Olsson GL 1994 Inhaled nitric oxide in neonates and children with pulmonary hypertension. Acta Paediatr 83: 1132–1136
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was funded by Grant 1 K23 HD41423–01 from the National Institute of Child and Human Development and supported by grants from the Sarnaik Endowment Fund, Children's Research Center of Michigan.The results reported in this manuscript were presented in part at the Pediatric Academic Societies' Annual Meeting on May 5, 2003 at Seattle, WA.Nebulizers were provided courtesy of Westmed, Inc., for part of the study.
Rights and permissions
About this article
Cite this article
Sood, B., Delaney-Black, V., Aranda, J. et al. Aerosolized PGE1: A Selective Pulmonary Vasodilator in Neonatal Hypoxemic Respiratory Failure Results of a Phase I/II Open Label Clinical Trial. Pediatr Res 56, 579–585 (2004). https://doi.org/10.1203/01.PDR.0000139927.86617.B6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000139927.86617.B6
This article is cited by
-
Hypoxia signaling in human health and diseases: implications and prospects for therapeutics
Signal Transduction and Targeted Therapy (2022)
-
Life-threatening PPHN refractory to nitric oxide: proposal for a rational therapeutic algorithm
European Journal of Pediatrics (2021)
-
Changes in vasoactive pathways in congenital diaphragmatic hernia associated pulmonary hypertension explain unresponsiveness to pharmacotherapy
Respiratory Research (2017)
-
Persistent pulmonary hypertension of the newborn
Maternal Health, Neonatology and Perinatology (2015)
-
Inhaled PGE1 in neonates with hypoxemic respiratory failure: two pilot feasibility randomized clinical trials
Trials (2014)