Abstract
Prolonged cerebral hypothermia is neuroprotective if started within a few hours of hypoxia-ischemia. However, delayed seizure activity is one of the major clinical indicators of an adverse prognosis after perinatal asphyxia. The aim of this study was to determine whether head cooling delayed until after the onset of postasphyxial seizures may still be neuroprotective. Unanesthetized near-term fetal sheep in utero received 30 min of cerebral ischemia induced by bilateral carotid artery occlusion. Eight and one-half hours later, they received either cooling (n = 5) or sham cooling (n = 13) until 72 h after the insult. Intrauterine cooling, induced by circulating cold water through a coil around the fetal head, was titrated to reduce fetal extradural temperature from 39.4 ± 0.1°C to between 30 and 33°C. Cerebral ischemia led to the delayed development of intense epileptiform activity from 6 to 8 h postinsult, followed by a marked secondary rise in cortical impedance (a measure of cytotoxic edema) and in carotid blood flow. Cerebral cooling markedly attenuated the secondary rise in impedance and reduced carotid blood flow (p < 0.001). After 5 d recovery, there was no significant difference in loss of parietal EEG activity relative to baseline in the hypothermia compared with the control group (- 12.5 ± 1.4 versus - 15.2 ± 1.2 dB, mean ± SEM, NS) or in parasagittal cortical neuronal loss (82 ± 9 versus 90 ± 5%, NS). In conclusion, delayed prolonged head cooling begun after the onset of postischemic seizures was not neuroprotective. These data highlight the importance of intervention in the latent phase, after reperfusion but before the onset of secondary injury.
Similar content being viewed by others
Main
Prolonged mild to moderate cerebral hypothermia initiated soon after hypoxia-ischemia has recently been shown to reduce neuronal loss in both immature(1–4) and adult animals(5,6). Such studies have led to the hypothesis that changes in postischemic cerebral temperature critically modulate encephalopathic processes, which evolve over time after the insult. As recently reviewed, whereas brief hypothermia immediately after resuscitation has limited and inconsistent results, extended periods of mild to moderate cerebral cooling are neuroprotective(7). Conversely, mild hyperthermia over a similar period is deleterious(6), and even very delayed episodes of hyperthermia may exacerbate injury after transient ischemia(8). These data are supported by the results of early clinical studies of therapeutic hypothermia(9–11). However, the width of the therapeutic window after which prolonged hypothermia is still protective remains poorly defined.
Clinical and experimental studies suggest that after a severe hypoxic-ischemic insult, cerebral energy metabolism, as measured by magnetic resonance spectroscopy, may transiently recover in a "latent" phase but then deteriorate from 6 to 15 h later in a secondary phase of cerebral energy failure(12,13). In infants, the degree of energy failure is correlated with adverse neurodevelopment at 1 and 4 y of age(13). In the near-term fetal sheep, 30 min of cerebral ischemia is associated with a secondary phase starting approximately 6 to 8 h after reperfusion. It is characterized by seizures, cytotoxic cell swelling, and accumulation of extracellular excitotoxins and is correlated with severe histologic injury(14–16). The EEG is initially profoundly suppressed in the latent phase after reperfusion, and there is transient postischemic hypoperfusion(1). From approximately 6 h after ischemia, EEG intensity begins to increase with a parallel rise in cerebral blood flow(1,17,18). The increase in EEG intensity is due to epileptiform activity with concurrent tonic-clonic activity(14,19).
In the newborn infant exposed to asphyxia, the appearance of delayed seizure activity is one of the major clinical indicators of an adverse prognosis, and, traditionally, therapy is directed to these infants(20). Thus it is important to determine whether therapeutic hypothermia may still be effective after the onset of seizures. We have previously reported that prolonged cerebral cooling started 90 min after severe cerebral ischemia in fetal sheep dramatically reduces neural injury(1), whereas cooling started at 5.5 h, shortly before the onset of epileptiform activity, is partially neuroprotective(18). The aim of the present study was to address the hypothesis that cerebral cooling delayed until shortly after the onset of postischemic epileptiform activity and maintained throughout the course of the secondary phase would alleviate cell membrane dysfunction and reduce secondary neuronal damage.
METHODS
In the present study, we used the approach of selectively cooling the head of fetal sheep in utero to minimize the systemic complications associated with whole body hypothermia(21). The use of a chronically instrumented fetal preparation to test the effect of postinsult cooling is clearly a compromise, as fetal and neonatal metabolism differ in several aspects. However, this approach provides an insult that occurs under fetal conditions(13), ensures stable control temperatures(22), and avoids experimental anesthesia that might interact with hypothermia(23).
Surgical procedures. Studies were approved by the Animal Ethics Committee of the University of Auckland. Romney/Suffolk fetal sheep from 117 to 124 d of gestation were operated on under 2% halothane/oxygen anesthesia as previously described(1,15). Polyvinyl catheters were inserted into both fetal brachial arteries and the amniotic sac. The vertebral-occipital anastomoses were ligated bilaterally to restrict vertebral blood supply to the carotid arteries; the lingual arteries were also ligated to restrict noncerebral blood flow(24). A double ballooned inflatable occluder cuff was placed around each carotid artery with a 3-mm ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY) just proximal to the cuff. Two pairs of EEG electrodes (AS633-5SSF, Cooner Wire Co., Chatsworth, CA) were placed on the dura over the parasagittal parietal cortex (5 mm and 15 mm anterior and 10 mm lateral to the bregma), with a reference electrode sewn over the occiput. To measure cortical impedance, a third pair of electrodes (Cooner Wire AS633-3SSF) was placed over the dura, 5 mm lateral to the EEG electrodes. A thermistor (Incu-Temp-1, Mallinckrodt Medical Inc., St. Louis, MO) was placed over the parasagittal dura 20 mm anterior to bregma. A 20-gauge catheter was placed in the sagittal sinus through a shallow midline burr hole. The burr holes were sealed, and the skin over the fetal skull was closed using cyanoacrylate glue.
A "cooling" coil made from silastic tubing (external diameter 7.9 mm, internal diameter 4.8 mm, Silclear, Degania Silicone, Israel) was attached over the dorsal surface of the scalp and extended over the lateral surface of the cranium down to the level of the external auditory meatus. A second thermistor (to measure fetal core body temperature) was placed in the fetal esophagus at the level of the right atrium(22). The fetus was then returned to the uterus, and all leads and catheters were exteriorized to the maternal flank. A polyvinyl catheter was placed in the maternal tarsal vein.
After the operation, the ewes were housed in metabolic cages at constant temperature (16 ± 1°C) and humidity (50 ± 10%) in a 12-h light/dark cycle and with free access to water and food. Gentamicin, 80 mg i.v., was administered daily to the ewe for 5 d starting at surgery.
Recordings. Fetal arterial blood pressure corrected for amniotic fluid pressure (Novatrans II, MX860, Medex Inc., OH), CaBF, fetal extradural temperature, and esophageal temperature as well as the fetal parietal EEG and impedance were recorded continuously. Recordings started at least 12 h before the experiment and continued for 120 h afterward. Signals were averaged at 1-min intervals and stored to disk by custom software (Labview for Windows, National Instruments Ltd., Austin, TX) running on an IBM compatible computer. The EEG signal was low pass filtered at 30 Hz, and then the intensity spectrum and impedance signal were extracted(1,15,17). The impedance of a tissue rises concomitantly as cells depolarize and fluid shifts from the extracellular to the intracellular space and, thus, is a measure of cytotoxic edema(15).
Experimental procedures. At least 3 d after surgery, reversible cerebral ischemia was induced by inflating the carotid cuffs bilaterally with sterile saline for 30 min. Successful occlusion was confirmed by the onset of an isoelectric EEG signal within 30 s of inflation. Fetal arterial and sagittal sinus blood samples were drawn 60 min before and just before occlusion and then at 35 min after the start of occlusion (i.e. 5 min after the end of occlusion) and at 2, 4, 6, 9, 9.5, 12, 24, 48, 72, 96, and 120 h. The arterial samples were analyzed for pH, Pao2, Pco2 (all corrected for core temperature), oxygen content (Ciba-Corning Diagnostics 845 blood gas analyzer and cooximeter, MA), and lactate and glucose levels (YSI model 2300, Yellow Springs, OH); oxygen content was also determined for the sagittal sinus samples to calculate the AVDO2. The average of the two preocclusion samples was taken as the baseline value for statistical analysis.
Fetuses were allocated to either cooling or sham cooling, starting at 9 h (8.5 h after reperfusion) and continuing until 72 h after occlusion. Ten of the fetuses were operated on in groups of two. Because of difficulties in obtaining sagittal sinus samples in two fetuses in the control group, data from eight additional previously studied control animals were added. These previous controls were identically instrumented and received sham cooling after the same carotid artery occlusion protocol(1,18). The histologic outcome of these eight sham cooled sheep was not significantly different from the five paired studies. Cooling was performed by linking the cooling coil over the fetal scalp with a pump in a cooled water bath. In the hypothermia group, the waterbath temperature was 6°C at the start of cooling and then was adjusted as necessary in the first 4 h to obtain an extradural temperature between 30 and 33°C. In sham cooled fetuses, the water was not circulated; thus the cooling coil and its contents remained in equilibrium with fetal temperature. The fetuses were monitored for 48 h after the end of cooling, and then the ewe and fetus were euthanized by barbiturate overdose.
Histologic analysis was performed as previously described(1,14). The fetal brain was perfusion fixed in situ with 10% phosphate buffered formalin. Predefined sections were selected according to the stereotaxic atlas for the near-term fetal sheep(25). Neuronal loss was scored by light microscopy on 8-µm thick coronal sections stained with thionin and acid fuchsin by two assessors, one of whom was blinded to the treatment group. There was good agreement between observers (r2 = 0.92). The proportion of neurons showing ischemic cell change in preassigned areas was scored on a 6-point scale: 0 = no dead neurons, 5 = >0-10%, 30 = >10-50%, 70 = >50-90%, 95 = 90-<100%, 100 = 100% dead neurons. Average scores were calculated for each region. The data were normalized by rank transformation, and the treatment groups compared by ANOVA.
Data analysis and statistics. Time series analysis of the post reperfusion data was performed off-line on the recorded signals. Average values for predetermined intervals were calculated: -12 to -6 h, -6 h to 0 min, then in 10-min intervals to 120 min, in 1-h intervals to 24 h, and then in 6-h intervals up to 120 h after carotid occlusion. Data have been plotted using the midpoint of each interval, except for the (6-h) baseline data that have been plotted at 0 h and the - 12 to -6-h data that are presented at -1 h.
The total EEG intensity (power) was normalized by log transformation [dB, 20 × log(intensity)]. The intensity was then normalized with respect to the baseline period: thus, all measurements are expressed as a ratio of baseline. The impedance data are presented as percentage of baseline and corrected for cerebral temperature(26). In each fetus, we measured the slope of impedance changes during a 30-min period of cooling to 30 to 33°C extradural temperature 24 h before the start of baseline recording and used it to correct the impedance signal for the effect of temperature changes during cooling: corrected impedance = impedance - (slope × Δtemperature). The correlation between temperature and impedance in each fetus was r2 ≥ 0.98.
CaBF was calculated as the sum of the left and right CaBF. Because the vertebrocarotid anastomoses were ligated, all blood flow to the cortex and midbrain structures was derived from the carotid circulation(27). CaBF was used an index of changes in global cerebral blood flow(24).
The effect of cooling on neuronal loss and other biophysical parameters was determined by ANOVA. Cerebral regions and time were treated as repeated measures to allow for repeated sampling. For comparisons over time, baseline levels were used as a covariate (ANCOVA). Where significant differences were found, post hoc comparisons of the means were made using the protected least significant difference test, adjusted using the baseline levels as the covariate, whereas changes relative to baseline were made with the Wilcoxon matched-pairs test. All data are presented as mean ± SEM.
RESULTS
Five fetal sheep were allocated to the 9-h cooling group and 13 to the sham cooling group. Data from eight of the sham cooling fetuses have previously been presented(1,18). There were no significant differences in any parameter between these eight and the five current fetuses. There were no significant differences between the hypothermia and sham cooled groups for mean gestational age at experiment (121.8 ± 1.0 versus 122.7 ± 0.9 d), fetal weight at postmortem (3864 ± 541 versus 3701.3 ± 185.2 g), baseline blood gases, pH, glucose, lactate, blood pressure, fetal heart rate, and CaBF.
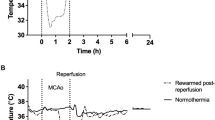
Cooling was associated with a significant fall in extradural temperature, reaching a nadir of 31.2 ± 0.2°C versus 39.3 ± 0.1°C (p < 0.001); the time sequence of changes is shown in the top panel of Figure 1. There was a smaller fall in esophageal temperature, to 37.1 ± 0.2°C, compared with a nadir of 39.2 ± 0.1°C in the sham cooled group (p < 0.001). The extradural and core temperatures in the hypothermia group remained significantly lower than in the sham cooled fetuses throughout the period of cooling (p < 0.001, repeated measures ANOVA).
Time sequence of changes in fetal temperature (t°), EEG intensity, and cortical impedance. The 30-min period of cerebral ischemia is shown by the vertical lines and solid bar, whereas cooling is shown by the shaded area. The top panel shows changes in extradural (•) and esophageal (▪) t° in the hypothermia group and extradural (○) and esophageal (□) t° in the sham cooled group. The lower two panels show changes in EEG intensity and cortical impedance (expressed as percentage of baseline) in the hypothermia (•) and sham cooled (○) groups. Mean ± SEM, ★p < 0.05 vs sham cooling, bar plus ★★p < 0.001 compared with sham cooling group (includes esophageal temperature).
There was a significant interaction between treatment group and time (p < 0.005) for fetal lactate and glucose but not for pH, Pao2, or Pco2. The hypothermia group showed a significantly greater rise (p < 0.05) in glucose at 24 h, from 0.7 ± 0.04 to 1.4 ± 0.2 mM, compared with the sham cooled group, 0.8 ± 0.07 to 1.0 ± 0.1 mM. Similarly, in the hypothermia group, lactate levels rose from 1.2 ± 0.07 at baseline to 2.7 ± 0.4 at 10 h and 2.8 ± 0.8 mM at 24 h, compared with the sham cooled group: 1.1 ± 0.07 at baseline, 2.5 ± 0.4 at 10 h (NS), and 1.3 ± 0.2 at 24 h (p < 0.05). Glucose (p < 0.05) but not lactate levels remained significantly elevated at 72 h in the hypothermia group (glucose 1.10 ± 0.08 versus 0.76 ± 0.06, p < 0.05; lactate 1.2 ± 0.1 versus 1.0 ± 0.05, NS). In both groups, all metabolic parameters had returned to normal at 96 and 120 h.
Bilateral carotid artery occlusion for 30 min resulted in a pattern of prolonged suppression of EEG activity after reperfusion, followed by a rapid transition to high intensity, low frequency epileptiform activity at approximately 6 to 8 h postinsult (Fig. 1, middle panel). The time sequence of changes in EEG intensity showed a significant interaction effect with treatment (ANOVA, p < 0.001). Although there seemed to be a small fall in EEG intensity immediately after the start of cooling at 9 h, this was not significant. Post hoc testing suggested that there was an extension of the phase of EEG hyperactivity in the hypothermia group compared with sham controls (Fig. 1). In both groups, EEG intensity ultimately declined to very low levels, and residual intensity in the final 48-h recovery period (after cessation of cooling) was not significantly different between the groups (p > 0.2, ANOVA).
The time sequence of changes in cortical impedance is shown in the bottom panel of Figure 1. Cortical impedance rose during occlusion, peaking at 166.0 ± 3.4 versus 156.1 ± 3.5% (hypothermia group versus sham cooled group, NS) 5 min after reperfusion, then almost fully resolved. In the sham cooled fetuses, impedance subsequently rose with the onset of epileptiform activity to a second peak of 139.4 ± 8.4% between 30 and 48 h. In contrast, the hypothermia group showed a much smaller attenuated secondary rise to 119.3 ± 3.4% of baseline (ANOVA, p < 0.001). This attenuated secondary peak was significantly higher than residual impedance levels (109.9 ± 2.1%, measured 120 min after reperfusion, p < 0.05).
The time sequence of changes in CaBF showed a significant interaction effect with treatment (Fig. 2, p < 0.001, ANOVA). CaBF transiently normalized after reperfusion; a period of secondary hypoperfusion then occurred between approximately 90 min and 6 h and resolved by 9 h. In the sham cooled group, a subsequent phase of hyperperfusion developed, peaking between 36 and 48 h, and then progressively resolved to below baseline levels. In contrast, the onset of cooling was associated with an immediate fall in CaBF, which then gradually normalized between 12 and 24 h despite continued cooling and then remained stable. CaBF was, thus, significantly reduced compared with sham cooled fetuses between 10 and 51 h (p < 0.01). From 66 to 96 h, CaBF was transiently higher in the hypothermia group (p < 0.05); however, there was no significant difference between the groups in the final 24 h of recovery.
Time sequence of changes in total CaBF, AVDO2, and MAP in the hypothermia (•) and sham cooled (○) groups. Cerebral ischemia is shown by the solid bar at the top of the graph, whereas cooling is shown by the shaded area as in Figure 1. There was a significant interaction between cooling and time on changes in CaBF (p < 0.001, repeated measures ANOVA). Mean ± SEM, ★p < 0.05 vs sham cooling. ★★p < 0.01 vs sham cooling. †p < 0.05 compared with baseline values.
The time course of changes in AVDO2 was not significantly different between the two groups (Fig. 2, p = 0.4, ANOVA). The only apparent difference in the time sequence of changes, on post hoc testing, was that there was a significant transient fall in cerebral oxygen difference at 24 h in the sham cooled fetuses (p < 0.01, compared with baseline) but not in the cooled group (p = 0.3, Fig. 2). There was an increase in AVDO2 5 min after reperfusion, followed by a fall that was maximal at 90 min after reperfusion (i.e. 120 min after the start of carotid occlusion; p < 0.001 for both groups combined). After 72 h, there was a further fall in AVDO2 in both groups, which was significantly lower than baseline levels at 96 h (p < 0.05 for both groups combined) and 120 h (p < 0.01 for both groups combined).
There was no significant effect of treatment on the time sequence of changes in fetal MAP (Fig. 2, bottom panel; p = 0.7). Both groups showed significant arterial hypertension during ischemia, which resolved after reperfusion. Subsequently, there was a further rise corresponding with the secondary rise in EEG activity (seizures). Although, as shown in Figure 2, hypothermia seemed to be associated with initial return to baseline MAP and then a small rise in MAP from 24 to 72 h, these trends did not reach significance. There was no significant effect of treatment on the time sequence of changes in fetal heart rate (data not shown, p = 0.8).
Thirty minutes of bilateral carotid arterial occlusion resulted in a watershed pattern of neuronal loss after 5 d of recovery. There was severe parasagittal cortical neuronal loss of 90.3 ± 4.5% in the sham cooled group, with no significant improvement, 82.0 ± 9.0%, in the hypothermia group. Overall, neuronal loss scores were not significantly lower in the hypothermia group compared with the sham cooled group (p = 0.11, repeated measures ANOVA, Fig. 3). Univariate analysis of each region separately suggested that neuronal loss may have been reduced in the striatum (p = 0.03). There was a trend to reduced neuronal loss in the dentate gyrus (p = 0.06) but not in cornu ammonis regions I (Fig. 3), III, or IV (data not shown) of the hippocampus.
Effect of cerebral cooling from 9 to 72 h after the start of ischemia on microscopically assessed neuronal loss in different brain regions 5 d after ischemia. No significant overall change (p = 0.11) in neuronal loss was seen in the hypothermia group (closed bars) compared with sham cooled fetuses (open bars). Univariate post hoc testing suggested the possibility of a small reduction in neuronal loss in the striatum. ★p < 0.05, Mann Whitney U test. Mean ± SEM.
DISCUSSION
Moderate selective cerebral hypothermia with mild systemic cooling starting 8.5 h after reperfusion from 30 min of cerebral ischemia and continuing for 3 d did not reduce overall neuronal death. These data are in contrast to the previously documented substantial neuroprotective effect of prolonged cerebral cooling initiated earlier after ischemia than in the present preparation(1,18). Interestingly, despite the ultimate lack of a neuroprotective effect, hypothermia markedly altered the evolution of the secondary phase, suppressing the delayed development of hyperperfusion and cytotoxic edema as measured by cortical impedance measurements. The lack of protective effect was not due to systemic compromise; hypotension did not occur, and only mild metabolic effects were seen, including transient rises in glucose and lactate. These systemic effects are consistent with the previously documented rise in catecholamines during cooling(22).
The timely selection of patients with hypoxic-ischemic encephalopathy for enrollment in a therapeutic trial presents formidable logistic difficulties(11). Many infants with severe acidosis or depressed Apgar scores at birth do not develop encephalopathy; thus, if such criteria are used, many infants will be treated unnecessarily. Possible methods of improving the selection of infants who might benefit from treatment include combining risk factors(28,29), early neurologic examination(11), and EEG recording(30). For example, background amplitude integrated EEG activity at 6 h is reported to be highly predictive of outcome(30); however, this technology is not yet universally available. Moreover, the predictive value of the common pattern of "burst suppression," which is associated with an intermediate outcome, is much lower(30). Clinically, one of the major prognostic factors associated with an adverse outcome is the development of postasphyxial seizures(29), and most clinicians would prefer to treat only such infants(20). It is, therefore, important to know whether therapy initiated after the onset of delayed seizures may be neuroprotective.
Previous studies suggested that the success of neuroprotection with hypothermia is determined by two key factors, the delay before initiating cooling and the duration of hypothermia. Significant neuroprotection has been reported despite delays in initiation of cooling of between 2 and 6 h, provided hypothermia was continued for a sufficient duration in relation to the severity of the insult; the optimum duration may be >48 h(5,31–33). The neuroprotective effect of prolonged cooling declines as the delay after ischemia is increased in both the adult and immature brain(1,5,18,31). In the same fetal sheep preparation used in the present study, cooling to an extradural temperature below 34°C, initiated 90 min after reperfusion and continued for 3 d, significantly improved recovery of EEG intensity with a concomitant substantial reduction in parasagittal cortical infarction and improvement in neuronal loss scores(1). Significant neuronal sparing still occurred when the delay was extended to 5.5 h after reperfusion, just before the onset of secondary seizures, although the effect was markedly reduced(18). The present data clearly show, however, that when cooling is delayed until after seizures are established, there is no electrophysiologic or overall histologic improvement. There was an indication of a possible treatment effect within the striatum and, to a lesser extent, the dentate gyrus of the hippocampus. This may be related to the relatively reduced injury of these regions in this paradigm; reduced severity has been associated with slower evolution of cell death and, hence, potentially a wider window for treatment(34).
The practical window of opportunity to start hypothermia after perinatal hypoxia-ischemia may be less than suggested from our experimental data, which are based on a single dense ischemic insult. In infants exposed to perinatal asphyxia, there may be considerable in utero evolution of injury as suggested, for example, by the presence of early seizures before 4 h of age(29). Further, the relationship between asphyxia and the onset of the secondary phase and seizures in man may differ from that seen experimentally. Nevertheless, there are now encouraging clinical data that also suggest neuroprotection with early hypothermia. After a stroke, lower mortality and better outcome are reported in patients with mild hypothermia on admission to hospital(35). Whole body cooling to 33°C initiated a mean of 74 min after out-of-hospital cardiac arrest and maintained for 12 h significantly improved outcomes compared with sequential controls(10). Finally, treatment with moderate whole body hypothermia for 24 h established a mean of 10 h after injury in adult patients with severe traumatic brain injury has recently been reported to hasten neurologic recovery and may have improved the long-term outcome(9).
The mechanism of delayed postinsult hypothermic neuroprotection is not known. The present data, taken with the results of our previous studies(1,18), clearly show that a key component of hypothermia is suppression of events in the latent phase after reperfusion and before the onset of the secondary phase, as shown by seizures and other pathophysiologic events. Thus, although cerebral cooling initiated immediately postinsult can, for example, reduce extracellular levels of excitotoxins and nitric oxide(36), these events resolve rapidly after reperfusion, and any subsequent elevation is confined to the secondary phase after the onset of seizures(16). It has been hypothesized that hypothermia acts by inhibiting events leading to programmed cell death (apoptosis); this is consistent with evidence that hypothermia reduces apoptosis after hypoxia-ischemia(3).
In the present study, the delay of 8.5 h in initiating cooling was chosen to follow the onset of seizures, and, thus, cooling was entirely restricted to the secondary phase. Surprisingly, despite significantly modifying several phenomena in the secondary phase, no overall protection was seen. Hypothermia was associated with a trend to a small immediate fall in EEG intensity and with significantly slower resolution of the secondary peak of activity; however, the final EEG intensity was not significantly higher than with sham cooled controls. Previous studies in this paradigm have shown that the final EEG intensity is related to the extent of neuronal loss in the underlying parasagittal cortex(14,15).
A rise in impedance directly reflects a shift of extracellular fluid into the cells causing cytotoxic edema(15). The timing of the secondary rise in the sham cooled fetuses, which followed the onset of delayed seizures, is comparable to the timing of secondary energy failure seen using magnetic resonance spectroscopy in infants after severe asphyxia(13). Hypothermia markedly attenuated this rise compared with sham cooling. However, in contrast to early cooling studies(1,18), a small secondary rise was still seen between 24 and 48 h. Other clinical(37) and experimental(38) studies confirm that hypothermia can prevent cytotoxic edema and reduce intracranial pressure. A direct effect to stabilize membrane function is suggested by in vitro data; hypothermia prevents intracellular ion and water entry and the consequent cell swelling despite inhibition of the ATP-dependent Na+/K+ pump by ouabain(39). The present data suggest, however, that attenuation of cytotoxic edema was not in itself neuroprotective. Further, the persistence of a small secondary peak, which was not seen when cooling was initiated earlier after the insult(1,18), suggests that a component of the antiedema effect in previous studies was indirectly mediated by suppression of cytotoxic processes during the latent phase.
We have previously shown that cerebral hypothermia initiated at 5.5 h extended the phase of secondary hypoperfusion to nearly 24 h after the insult. In the present study, cooling was initiated after resolution of this phase; during cooling, there was a further fall in CaBF to levels comparable to the previous study, which was not due to a fall in fetal blood pressure. Despite continued cooling, CaBF then progressively returned to baseline levels by 24 h. Although CaBF is only an index of cerebral blood flow(24), these data are consistent with suppression of global cerebral metabolism during the secondary phase compared with sham cooling(40) and, thus, suggest that suppression of metabolism during the secondary phase is not intrinsically neuroprotective.
There was a significant transient fall in AVDO2 in the sham cooled but not the cooled group at 24 h; this time point corresponded with the peak of the phase of secondary hyperperfusion. This suggests that this rise in CaBF represented a true "luxury" perfusion, greater than the requirements of the brain. Consistent with this, a similar pattern of a delayed rise in cerebral blood volume has been previously documented using near-infrared spectroscopy of the brain(17). In the last 48 h of the present study, after rewarming, CaBF and EEG intensity were stable in both groups and similar to baseline levels. Despite this, AVDO2 fell further to significantly below baseline, consistent with ongoing impairment of cerebral metabolism.
In conclusion, after a severe ischemic insult in fetal sheep, moderate cerebral hypothermia delayed until shortly after the onset of postischemic seizures and then maintained throughout the secondary phase did not significantly improve global neural outcome. Hypothermia alleviated secondary cell membrane dysfunction and prevented the phase of secondary hyperperfusion but did not improve electrophysiologic or histologic recovery in the parasagittal watershed region. These data highlight the importance of intervention in the latent phase after reperfusion, before the onset of secondary injury as shown by seizures and other pathologic processes. There is a need for further studies of the mechanisms of injury in this phase that precedes irreversible delayed cell death.
Abbreviations
- dB:
-
decibels
- CaBF:
-
carotid blood flow
- AVDO2:
-
cerebral arteriovenous difference for oxygen
- MAP:
-
mean arterial blood pressure
References
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD 1997 Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal sheep. J Clin Invest 99: 248–256.
Sirimanne ES, Blumberg RM, Bossano D, Gunning MI, Edwards AD, Gluckman PD, Williams CE 1996 The effect of prolonged modification of cerebral temperature on outcome following hypoxic ischemic injury in the infant rat. Pediatr Res 39: 591–598.
Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, Cooper CE, Wyatt JS, Reynolds EO, Mehmet H 1995 Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun 217: 1193–1199.
Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M 1998 Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res 43: 738–745.
Colbourne F, Corbett D 1995 Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci 15: 7250–7260.
Coimbra C, Drake M, Boris-Moller F, Wieloch T 1996 Long-lasting neuroprotective effect of postischemic hypothermia and treatment with an anti-inflammatory/antipyretic drug. Evidence for chronic encephalopathic processes following ischemia. Stroke 27: 1578–1585.
Gunn AJ, Gunn TR 1998 The "pharmacology" of neuronal rescue with cerebral hypothermia. Early Hum Dev 53: 19–35.
Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD 1996 Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke 27: 2274–2280.
Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST 1997 Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 336: 540–546.
Bernard SA, Jones BM, Horne MK 1997 Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 30: 146–153.
Gunn AJ, Gluckman PD, Gunn TR 1998 Selective head cooling in newborn infants following perinatal asphyxia: a safety study. Pediatrics 102: 885–992.
Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS, Reynolds EO 1995 Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 37: 667–670.
Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, Reynolds EOR, Stewart AL 1997 Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol 39: 718–725.
Williams CE, Gunn AJ, Synek B, Gluckman PD 1990 Delayed seizures occurring with hypoxic-ischemic encephalopathy in the fetal sheep. Pediatr Res 27: 561–565.
Williams CE, Gunn AJ, Gluckman PD 1991 The time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 22: 516–521.
Tan WKM, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckman PD 1996 Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res 39: 791–797.
Marks KA, Mallard EC, Roberts I, Williams CE, Sirimanne ES, Johnston BM, Gluckman PD, Edwards AD 1996 Delayed vasodilation and altered oxygenation following cerebral ischemia in fetal sheep. Pediatr Res 39: 48–54.
Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD 1998 Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 102: 1098–1106.
Tan WKM, Williams CE, Gunn AJ, Mallard EC, Gluckman PD 1992 Suppression of postischemic epileptiform activity with MK-801 improves neural outcome in fetal sheep. Ann Neurol 32: 677–682.
Connell J, Oozeer R, de Vries L, Dubowitz LMS, Dubowitz V 1989 Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child 64: 459–464.
Schubert A 1995 Side effects of mild hypothermia. J Neurosurg Anesthesiol 7: 139–147.
Gunn TR, Butler JH, Gluckman PD 1986 Metabolic and hormonal responses to cooling the fetal lamb in utero. J Dev Physiol 8: 55–66.
Nakashima K, Todd MM 1996 Effects of hypothermia, pentobarbital, and isoflurane on postdepolarization amino acid release during complete global cerebral ischemia. Anesthesiology 85: 161–168.
Van Bel F, Roman C, Klautz RJM, Teitel DF, Rudolph AM 1994 Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr Res 35: 329–333.
Gluckman PD, Parsons Y 1983 Stereotaxic method and atlas for the ovine fetal forebrain. J Dev Physiol 5: 101–128.
Smith DC 1992 Effects of skin blood flow and temperature on skin-electrode impedance and offset potential: measurements at low alternating current density. J Med Eng Technol 16: 112–116.
Baldwin BA, Bell F 1963 The anatomy of the cerebral circulation in the sheep and ox. The dynamic distribution of the blood supply by the carotid and vertebral arteries to cranial regions. J Anat 97: 203–215.
Perlman JM, Risser R 1996 Can asphyxiated infants at risk for neonatal seizures be rapidly identified by current high-risk markers?. Pediatrics 97: 456–462.
Ekert P, Perlman M, Steinlin M, Hao Y 1997 Predicting the outcome of postasphyxial hypoxic-ischemic encephalopathy within 4 hours of birth. J Pediatr 131: 613–617.
Hellstrom Westas L, Rosen I, Svenningsen NW 1995 Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full-term infants. Arch Dis Child 72: F34–F38.
Coimbra C, Wieloch T 1994 Moderate hypothermia mitigates neuronal damage in the rat brain when initiated several hours following transient cerebral ischemia. Acta Neuropathol 87: 325–331.
Carroll M, Beek O 1992 Protection against hippocampal CA1 cell loss by postischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis 7: 45–50.
Colbourne F, Auer RN, Sutherland GR 1998 Characterization of postischemic behavioral deficits in gerbils with and without hypothermic neuroprotection. Brain Res 803: 69–78.
Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD 1995 Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Mol Brain Res 29: 1–14.
Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, Olsen TS 1996 Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 347: 422–425.
Thoresen M, Satas S, Pukasundvall M, Whitelaw A, Hallstrom A, Loberg EM, Ungerstedt U, Steen PA, Hagberg H 1997 Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 8: 3359–3362.
Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W 1997 Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology 48: 762–767.
Ebmeyer U, Safar P, Radovsky A, Obrist W, Alexander H, Pomeranz S 1998 Moderate hypothermia for 48 hours after temporary epidural brain compression injury in a canine outcome model. J Neurotrauma 15: 323–336.
Zeevalk GD, Nicklas WJ 1996 Hypothermia and metabolic stress-narrowing the cellular site of early neuroprotection. J Pharmacol Exp Ther 279: 332–339.
Laptook AR, Corbett RJT, Sterett R, Garcia D, Tollefsbol G 1995 Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res 38: 919–925.
Acknowledgements
This study is dedicated to the memory and work of Professor Tania Gunn.
Author information
Authors and Affiliations
Additional information
Supported by USPHS grant R01 HD-32752, the Auckland Medical Research Foundation, the New Zealand Lottery Grants Board, and the Health Research Council of New Zealand.
Rights and permissions
About this article
Cite this article
Gunn, A., Bennet, L., Gunning, M. et al. Cerebral Hypothermia Is Not Neuroprotective When Started after Postischemic Seizures in Fetal Sheep. Pediatr Res 46, 274–280 (1999). https://doi.org/10.1203/00006450-199909000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199909000-00005
This article is cited by
-
Regional variability in therapeutic hypothermia eligibility criteria for neonatal hypoxic-ischemic encephalopathy
Pediatric Research (2024)
-
Multichannel EEG abnormalities during the first 6 hours in infants with mild hypoxic–ischaemic encephalopathy
Pediatric Research (2021)
-
Characteristics and short-term outcomes of neonates with mild hypoxic-ischemic encephalopathy treated with hypothermia
Journal of Perinatology (2020)
-
Diffusion kurtosis imaging and pathological comparison of early hypoxic–ischemic brain damage in newborn piglets
Scientific Reports (2020)
-
Severity of hypoxic ischemic encephalopathy and heart rate variability in neonates: a systematic review
BMC Pediatrics (2019)