Abstract
Purpose: The purpose of this literature review is to report primary care physicians’ perceived barriers concerning the provision of genetic services.
Methods: Sixty-eight papers were identified in six electronic databases. Only publications classified as empirical studies (N = 18) were included in this review.
Results: Barriers identified most frequently in reviewed studies were inadequate knowledge of basic genetics, lack of detailed or updated family histories, lack of confidence, and lack of referral guidelines.
Conclusion: Although many primary care physicians perceive genetics as a low practice priority, they do report a need for educational programs in genetics, informational resources, and referral guidelines.
Similar content being viewed by others
Main
The achievements of the Human Genome Project have enabled researchers to identify errors in genes that may either cause or contribute to disease. As a result of the expansion of genomics into human health applications, an increasing number of gene tests are becoming available commercially.1 These tests will play an important role in the diagnosis, monitoring, and treatment of diseases.
According to the director of the National Human Genome Research Institute, Francis Collins, MD, PhD, “This ‘next revolution in medicine’ will fall on the shoulders of physicians who provide primary care.”2(p1) While many primary care providers (PCPs) already incorporate genetic screening into their routine services, it is reasonable to assume that the demands on family physicians will increase substantially as they are required to provide information on new genetic tests to their patients, to help interpret test results, and to consider prescribing new genetic therapies as they become available. One fundamental challenge that PCPs will encounter is becoming familiar with an ever increasing number of technologies for both screening and treatment of genetic disorders.
If Collins’ prediction is true, questions regarding PCPs’ willingness, capacity, and resources to provide genetic services inevitably arise. The review of literature presented here represents an attempt to address some of these questions.
This review systematically examines and organizes findings from available studies of PCPs’ views regarding the provision of genetic services. The specific research question guiding this review is, What are PCPs’ perceived barriers that hinder the routine provision of genetic services? For the purposes of this review, genetic services are defined as patient-oriented tasks that include (but are not limited to) screening for treatment and prevention, counseling regarding genetic testing results, referring patients with genetic risks, and comprehensive family history-taking. In addition to answering the proposed question, recommendations from the literature regarding knowledge, skills, and resources needed to overcome these barriers also are examined. In the context of this review, PCPs include family or general practitioners, internists, obstetricians and gynecologists, as well as pediatricians.
METHODS
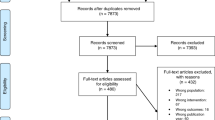
A search of six electronic databases from the decade after the beginning of the Human Genome Project, 1991 to 2001, revealed 68 papers that investigated or discussed the provision of genetic services by primary care physicians. The databases searched were Medline, ERIC, PsycINFO, Healthstar, Cancerlit, and Annual Reviews. Key terms combined with “genetics,” “genetic testing,” or “genetic screening” through the Boolean term “and” included “primary care,” “primary care providers,” “primary care physicians,” “family practice,” and “general practitioners.” Reference lists from retrieved papers were also examined.
Eighteen of the 68 papers examined were classified as research studies. The 50 excluded papers were commentaries, theoretical, or otherwise nonempirical examinations of the issue. The 18 included studies were written in the English language and published in peer-reviewed journals. Their focus, methods, and outcomes varied. Studies that focused on proposed solutions to previously identified barriers were also included. The studies were carried out in the United States (9), United Kingdom (6), Scotland (1), the Netherlands (1), and Switzerland (1). A review matrix was created to structure information abstracted from each study.3 Table 1 is an adaptation of that original matrix.
Studies also were rated according to their methodological quality. The rating was determined by using the set of criteria and codes developed by Bernstein and Freeman4 and described by Patton.5 The design of these criteria is based on assumptions that value quantitative, controlled studies, over qualitative efforts. The criteria cover the dimensions of sampling, data analysis, statistical procedures, and design. For each of these, a numbered score was given to the reviewed study if it contained the specific elements defined for each dimension. For instance, if the sampling procedure involved systematic random sampling, the study received a score of 1; if the sample was nonrandom, cluster, or nonsystematic, it received a score of 0. The maximum score a study could obtain when all criteria were used was 10. A score of 10 indicates that the study utilized a systematic random sample, it was quantitative in nature, it utilized multivariate statistical analyses, and its design consisted of experimental or quasi-experimental randomization with control groups.
RESULTS
Table 2 summarizes the barriers encountered in the reviewed studies. In the text below, only barriers identified in three or more studies are discussed.
Lack of genetic knowledge
Twelve of the 18 studies identified inadequate knowledge of genetics, genetic testing, or genetic counseling as a barrier to providing genetic information or services (Table 2). From a national survey of family practitioners, obstetricians, gynecologists, internists, and psychiatrists, Hofman and colleagues6 found recent medical school graduates appeared to have a higher level of genetic knowledge. This suggests medical schools are incorporating more genetic education into the curriculum. Teague and colleagues7 tested a medical education module on medical students to assess their pretest and posttest knowledge of and attitudes toward cancer and predictive genetic testing. After hearing discussions led by genetic specialists of case examples, medical students seemed more favorable toward genetic testing and genetic counseling.
Kolb and colleagues8 estimated that the majority of underutilization of genetic services by PCPs was due to lack of adequate genetics information and knowledge. They found a significant increase in knowledge and attitudes of PCPs in a Texas community toward the provision of prenatal and children’s genetic services after a 16-hour basic genetic educational program. A 150-page course manual, several pamphlets on specific genetic conditions, and a videotape that was developed by the Texas Department of Health accompanied the program. As an aid to the knowledge barrier, a Texas survey of physicians’ attitudes and practices of cancer genetics revealed physicians would like to see a variety of continuing education programs and educational materials on DNA testing for cancer susceptibility.9
In a qualitative study, Kumar and Gantley10 reported general practitioners in Britain view “new genetics” as a series of additional tasks requiring new knowledge and skills, rather than an extension of current practice. Focus groups with 26 general practitioners in Britain11 also revealed lack of genetic knowledge and referral skills as a barrier to providing genetic services, although these physicians perceived genetics as an important and increasingly relevant topic for primary care.
Positive attitudes toward genetic testing for breast and ovarian cancer were found in Escher and Sappino’s12 study but, again, knowledge deficits underlined a need for genetic education. Watson et al.13 found many general practitioners were unsure about their decisions to refer patients with a high risk for breast cancer and were uncertain about how to manage lower-risk patients who were not referred.
Mountcastle-Shah and Holtzman14 believe skepticism about the impact of genetic discoveries on primary care practice could be a barrier in the provision of genetic services. Because of this skepticism, genetic training is a low priority for many primary care physicians.15,16 Six percent of physicians in Hayflick and colleagues’16 study reported no need for additional genetic services. Some of the general practitioners in Kumar and Gantley’s qualitative study felt genetic advances had little relevance for their practice: “Genetic conditions are not our bread and butter; the new genetics has little impact on my day-to-day clinical work.”10(p1411)
Mountcastle-Shah and Holtzman14 proposed encouraging primary care physicians to participate in clinical studies to assess the safety and effectiveness of genetic testing. They believe participation in research may serve as an effective medium for physician education in genetics.
A pretested survey to measure hereditary breast cancer knowledge and practice behavior was mailed to a random sample of 400 family practitioners in Denver, Colorado. Mouchawar et al.17 reported a low knowledge level relating to genetic principles in general and hereditary breast cancer in particular, even though 100% of the surveyed physicians took family cancer histories as part of routine clinical practice.
Lack of detailed or updated family history
Limited time to obtain a detailed family history was a barrier in 4 of the 18 studies reviewed.11,15,18,19 Use of family histories has been erratic for most primary care practices. An accurate family history is useful to make a diagnosis of a genetic disease, to determine a person’s risk of developing a genetic disease, or to determine the risk of having a child with a genetic disease. Despite its utility, time availability limits the extent of history-taking by PCPs. As one physician pointed out, “…I’ve no idea how long a genetic history would take but if you were to add that on to the day I don’t know how that would be resourced….”11(p422)
In a survey of Alabama primary care physicians, 71% of respondents obtained family histories from new patients, but nearly half did not update them at annual examinations.18 In this study, the information obtained about family cancer history was less than optimal for genetic risk assessment, screening, and prevention. Acheson and colleagues19 also found family histories were updated less than half the time during an established patient’s visit.
Lack of referral guidelines
Lack of guidelines was identified as a barrier in three of the reviewed studies.15,20,21 Fry and colleagues affirmed that estimation of genetic cancer risk based on family history “is an unreliable method by which to expect general practitioners to make appropriate referrals to regional services.”15(p473) These authors felt guidelines supportive of general practitioners’ referral decisions need to be provided. De Bock and colleagues20 attempted to develop a set of simple, practical guidelines for primary care physicians to use for referring and counseling women with a family history of breast cancer. While the simple guidelines lowered the number of misclassified patients, the researchers felt more testing and refining was needed to increase the guideline’s sensitivity for referral to a family cancer clinic.
After discussion with local general practitioners, surgeons, radiologists, gynecologists, public health physicians, and geneticists, Lucassen and colleagues21 found PCPs have difficulty with genetic referral decisions unless the risk is either very low or very high. These researchers established guidelines for referral of patients to a family cancer clinic and found that by using their practical guidelines, fewer “lower risk” patients were referred. In addition, analysis of general practitioners’ letters of referral showed agreement with the genetic clinic’s risk assessment.
Lack of confidence
Assessing and counseling about genetic risks requires knowing which choices are available. Watson and colleagues22 found general practitioners lacked information about genetic services and options available to patients which, in turn, decreased their confidence to refer patients to services offered by genetic clinics. Authors of that study sought to determine whether provision of printed materials alone was effective to disseminate new knowledge and implement guidelines successfully. In their study, one group of physicians was issued a tailored information pack while another group received an education session and an information pack. No additional improvements in referral decisions were seen when general practitioners received an educational session along with the information pack. However, both groups fared better, with regard to appropriate referrals, than the control group that did not receive either aid.
Emery and colleagues23 tested a computer program for assessing genetic risk of cancer in primary care. Before exposure to the computer program, general practitioners stated that while they felt it was their role to assess and refer patients with genetic risks, most felt uncomfortable with the task, even after attending courses on cancer genetics. According to the authors, “The doctors managed this discomfort in a variety of ways: some referred all patients concerned about their family history of cancer, some assessed risk using a heuristic approach, reflecting an incomplete memory of referral guidelines, and others attempted to reassure patients in the face of uncertainty.”23(p34)
Fry and colleagues15 assessed the views of 397 general practitioners in Scotland, regarding their role in cancer genetics services and their confidence in carrying out that role. The study’s findings revealed low levels of confidence, even for genetic services tasks thought to be part of general practitioners’ role. For instance, only 27% of respondents felt “confident, or very confident” “deciding which patients should be referred to a regional cancer genetics clinic.”15(p471)
Methodological quality
Table 1 displays each study’s methodological quality score, out of the possible maximum score of 10. Given the bias inherent in the criteria, the lowest-ranking studies were those that used a qualitative methodology. Nevertheless, qualitative studies were carried out with rigor and concern for validity and reliability. Of interest, one of the qualitative studies14 achieved—even when compared to quantitative criteria—a score of 6. This study had a randomly selected sample and used both qualitative and quantitative analyses.
The methodological quality scores for the quantitative studies ranged from 3 to 9, with an average overall quality rating of 5.47. Only three of the studies6,8,18 mentioned any validation of their survey instrument, or assessment of the scores’ reliability.24 Ten of the studies used descriptive statistics exclusively,7–9,11–13,15–18 while only two studies used multivariate statistical analyses.6,19
DISCUSSION
According to this review, the majority of studies found that many health care providers are uncertain whether genetic discoveries will impact the daily practice of medicine in their primary care setting. For those PCPs who are willing to meet the rising demand for genetic health care, however, additional education and training in basic genetics will become an important need. According to Greendale and Pyeritz,25 most of currently practicing physicians were exposed to only 29 hours of genetics in medical school and most of that knowledge is outdated. Williams complained that despite the “explosion of genetic knowledge in the past ten years,” time devoted to genetics in medical school curricula remains the same.26(p434)
Although exposure to formal training in genetics within medical school curricula may affect only recently trained students, interventions are being designed to reach practicing providers. Evaluations of information resources designed to aid practicing physicians with the provision of genetic services are also beginning to emerge within the literature. These evaluations are finding that most physicians obtain genetic information from medical journals, continuing medical education courses, other practitioners, professional meetings, or pharmaceutical contacts.6 Regarding the diffusion of genetic knowledge in primary care, a physician in Mountcastle-Shah and Holtzman’s study stated, “Well it’s going to be analogous to dumping the Encyclopedia Britannica on a ten-year-old. We’re going to be able to read it, we’re going to have a lot of information there.…The largest part of the challenge is going to be making sense out of it and deciding how we use it—making sense of its utility if you will.”14O(p413)
Although lack of knowledge was the most often cited barrier among the reviewed publications, studies that identified lack of knowledge as a barrier operated under a basic and, perhaps, problematic assumption: that PCPs’ lack of knowledge and/or skepticism is undesirable. Instead, studies of PCPs’ knowledge levels would do well to examine the meaning of this void, and consider whether ignorance about genetic developments may signify, instead, uncertainty about the clinical utility of specific technologies. Such valid uncertainty or skepticism is not unique to PCPs, and it is equally shared by geneticists, practitioners, and public health professionals. As Burke and colleagues affirm, “When a [genetic] test has poor ability to predict clinical outcome and there is no associated treatment, testing is difficult to justify on either medical or social grounds.”27(p238) Interventions designed to increase PCPs’ genetic knowledge, therefore, should incorporate debate about genetic tests’ clinical validity and treatment effectiveness.27
Findings from this review also suggest that—along with knowledge acquisition—PCPs will require training for the refinement of specific skills such as family history-taking and use of referral guidelines. Undoubtedly, family history represents a strong risk factor in many diseases28; it remains unclear, however, what types of family history and which elements of family history-taking are most appropriate in a primary care setting. Further investigation into these issues is essential.
Along with appropriate family history-taking, the availability of and appropriate training in the use of referral guidelines also warrant attention. A referral to a genetic clinic is expensive20 and can cause undue stress and anxiety21 for the patient and his/her family. As referral guidelines are being developed to assess and refer high-risk cases to genetic services, more care should be placed upon the development of guidelines for the management of low- and moderate-risk patients in a primary care setting.
The mere availability of appropriate and simple guidelines, however, does not guarantee that providers will feel comfortable using them routinely. Self-efficacy, or a level of confidence that one has the skills, resources, and persistence to competently perform screening and referral tasks, will also influence the decision to incorporate these services into practice. As seen in this review’s findings, lack of confidence may represent an important barrier that must be addressed along with knowledge and specialized skills. Further examination of these two barriers (lack of referral guidelines and low perceived self-efficacy to perform genetics-related tasks) should consider the compounded effect of one barrier on the other, in place of examining each of them in isolation: to what extent, for instance, is lack of confidence (self-efficacy) related to the absence of clearly outlined guidelines and, conversely, to what extent is the absence of guidelines due to assumptions about physicians’ self-efficacy to screen for genetic risks?
While this study’s main strength lies in organizing the available empirical evidence concerning PCPs’ perceptions of barriers to the provision of genetic services, its main weakness is found precisely in the data being organized. None of the studies reviewed here (with one exception14) were designed to primarily assess providers’ perceived barriers; such barriers were usually captured in the course of measuring other factors such as physicians’ knowledge, attitudes, perceptions of role vis-à-vis genetic technology, referral behavior, and levels of confidence with specific genetic-services practices. Given that 7 of the 18 reviewed studies were designed to assess PCPs’ knowledge, it is not unexpected to find that knowledge-related factors were the most often cited barriers limiting a more extensive inclusion of genetic technologies into primary care practice.
Another important limitation of this review is the methodological quality of the studies summarized. Examination of these studies’ quality scores reveals that this body of literature is composed, mainly, of noninferential analyses of convenience samples with infrequent reporting of validity of measures and reliability of findings. Ten of the reviewed studies, for instance, had nonrandom, cluster, or nonsystematic samples. Despite the clear bias in favor of quantitative methods inherent in the evaluation criteria used in this review, some of the most useful information was provided by four qualitative studies.10,11,14,23 Qualitative data provided richer descriptions of respondents’ views and identified issues that were relevant to respondents’ professional groups. Findings from this study suggest it is paramount that future research on PCPs’ incorporation of genetic technologies pay attention to the quality of a study’s design and measures, as well as strive to use valid methods and collect reliable data utilizing quantitative, qualitative, or mixed-methods approaches.
In addition to the need for methodological rigor, this review’s findings suggest that further studies are needed to fill two important gaps. The first gap relates to the need for validation of these studies’ findings and for establishment of their generalizability to various populations of PCPs, especially in nations other than Britain and the United States. The second gap consists of the need for evaluation studies of targeted interventions that address the barriers identified thus far. If lack of knowledge, for instance, remains an important barrier (after further validation studies), evaluation research is needed to determine which interventions are best suited to overcome this barrier: are continuing education credits better than conference presentations, for instance? Along with evaluation studies, further examination of which factors are perceived as facilitators of providers’ behaviors are also needed. The identification and evaluation of factors amenable to intervention for supporting PCPs in their future genetics-related tasks may provide useful tools to promote the integration of genetics and primary care.
References
Human Genome Management Information System, 2001. http://www.ornl.gov/hgmis/project/about.html.
American Academy of Family Physicians, 1998. http://www.aafp.org/fpr/981100fr/2.html.
Garrard J . Health sciences literature review made easy: the matrix method. Gaithersburg, MD: Aspen, 1999.
Bernstein I, Freeman HE . Academic and entrepreneurial research: consequences of diversity in federal evaluation studies. New York: Russell Sage, 1975.
Patton MQ . Utilization-focused evaluation: the new century text, 3rd ed. Thousand Oaks, CA: Sage Publications, 1997.
Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA . Physicians’ knowledge of genetics and genetic tests. Acad Med 1993; 68: 625–632.
Teague KE, Brown JA, Meyer JM, Ellis Kahn MJ, Smith TJ, Kreutzer KO et al. Teaching efficacy of a medical education module on genetic testing for cancer. J Cancer Educ 1996; 11: 196–202.
Kolb SE, Aguilar MC, Dinenberg M, Kaye CI . Genetics education for primary care providers in community health settings. J Community Health 1999; 24: 45–59.
Friedman LC, Plon SE, Cooper HP, Weinberg AD . Cancer genetics-survey of primary care physicians’ attitudes and practices. J Cancer Educ 1997; 12: 199–203.
Kumar S, Gantley M . Tensions between policy makers and general practitioners in implementing new genetics: grounded theory interview study. BMJ 1999; 319: 1410–1413.
Watson EK, Shickle D, Qureshi N, Emery J, Austoker J . The “new genetics” and primary care: GPs’ views on their role and their educational needs. Fam Pract 1999; 16: 420–425.
Escher M, Sappino AP . Primary care physicians’ knowledge and attitudes towards genetic testing for breast-ovarian cancer predisposition. Ann Oncol 2000; 11: 1131–1135.
Watson E, Austoker J, Lucassen A . A study of GP referrals to a family cancer clinic for breast/ovarian cancer. Fam Pract 2001; 18: 131–134.
Mountcastle-Shah E, Holtzman NA . Primary care physicians’ perceptions of barriers to genetic testing and their willingness to participate in research. Am J Med Genet 2000; 94: 409–416.
Fry A, Campbell H, Gudmunsdottir H, Rush R, Porteous M, Gorman D et al. GPs’ views on their role in cancer genetics services and current practice. Fam Pract 1999; 16: 468–474.
Hayflick SJ, Eiff MP, Carpenter L, Steinberger J . Primary care physicians’ utilization and perceptions of genetics services. Genet Med 1998; 1: 13–21.
Mouchawar J, Klein CE, Mullineaux L . Colorado family physicians’ knowledge of hereditary breast cancer and related practice. J Cancer Educ 2001; 16: 33–37.
Acton RT, Burst NM, Casebee L, Ferguson SM, Green P, Laird BL et al. Knowledge, attitudes, and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med 2000; 75: 850–852.
Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC . Family history-taking in community family practice: implications for genetic screening. Genet Med 2000; 2: 180–185.
de Bock GH, Vliet Vlieland TPM, Hageman GCHA, Oosterwijk JC, Springer MP, Kievit J . The assessment of genetic risk of breast cancer: a set of GP guidelines. Fam Pract 1999; 16: 71–77.
Lucassen A, Watson E, Harcourt J, Rose P, O’Grady J . Guidelines for referral to a regional genetics service: GPs respond by referring more appropriate cases. Fam Pract 2001; 18: 135–140.
Watson E, Clements A, Yudkin P, Rose P, Bukach C, Mackay J et al. Evaluation of the impact of two educational interventions on GP management of familial breast/ovarian cancer cases: a cluster randomized controlled trial. Br J Gen Pract 2001; 51: 817–821.
Emery J, Walton R, Coulson A, Glasspool D, Ziebland S, Fox J . Computer support for recording and interpreting family histories of breast and ovarian cancer in primary care (RAGs): qualitative evaluation with simulated patients. BMJ 1999; 319: 32–36.
DeVellis RF . Scale development: theory and applications. Applied social research methods series, volume 26. Newbury Park, CA: Sage Publications, 1991.
Greendale K, Pyeritz RE . Empowering primary care health professionals in medical genetics: How soon? How fast? How far? Am J Med Genet 2001; 106: 223–232.
Williams MS . Genetics and managed care: policy statement of the American College of Medical Genetics. Genet Med 2001; 3: 430–435.
Burke W, Pinsky LE, Press NA . Categorizing genetic tests to identify their ethical, legal, and social implications. Am J Med Genet 2001; 106: 233–240.
Emery J, Lucassen A, Murphy M . Common hereditary cancers and implications for primary care. Lancet 2001; 358: 56–63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suther, S., Goodson, P. Barriers to the provision of genetic services by primary care physicians: A systematic review of the literature. Genet Med 5, 70–76 (2003). https://doi.org/10.1097/00125817-200303000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1097/00125817-200303000-00004