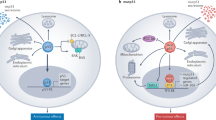
Abstract
The p53 tumor suppressor gene is the most frequently mutated gene in cancer. Most p53 mutations are missense point mutations that cluster in the DNA-binding core domain. This results in distortion of core domain folding and disruption of DNA binding and transcriptional transactivation of p53 target genes. Structural studies have demonstrated that mutant p53 core domain unfolding is not irreversible. Mutant p53 is expressed at high levels in many tumors. Therefore, mutant p53 is a promising target for novel cancer therapy. Mutant p53 reactivation will restore p53-dependent apoptosis, resulting in efficient removal of tumor cells. A number of strategies for targeting mutant p53 have been designed, including peptides and small molecules that restore the active conformation and DNA binding to mutant p53 and induce p53-dependent suppression of tumor cell growth in vitro and in vivo. This opens possibilities for the clinical application of mutant p53 reactivation in the treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abarzúa P, LoSardo JE, Gubler ML, Neri A . (1995). Restoration of the transcription activation function to mutant p53 in human cancer cells. Cancer Res 55: 3490–3494.
Ang HC, Joerger AC, Mayer S, Fersht AR . (2006). Effects of common cancer mutations on stability and DNA binding of full-length p53 compared with isolated core domains. J Biol Chem 281: 21934–21941.
Bargonetti J, Manfredi JJ, Chen X, Marshak DR, Prives C . (1993). A proteolytic fragment from the central region of p53 has marked sequence-specific DNA-binding activity when generated from wild-type but not from oncogenic mutant p53 protein. Genes Dev 7: 2565–2574.
Bell S, Klein C, Muller L, Hansen S, Buchner J . (2002). p53 contains large unstructured regions in its native state. J Mol Biol 322: 917–927.
Bensaad K, Le Braas M, Unsal K, Strano S, Blandino G, Tominaga O et al. (2003). Change of conformation of the DNA-binding domain of p53 is the only key element for binding of and interference with p73. J Biol Chem 278: 10546–10555.
Béroud C, Soussi T . (1998). p53 gene mutation: software and database. Nucleic Acids Res 26: 200–204.
Blagosklonny MV, Toretsky J, Bohen S, Neckers L . (1996). Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci USA 93: 8379–8383.
Brachmann RK, Yu K, Eby Y, Pavletich NP, Boeke JD . (1998). Genetic selection of intragenic suppressor mutations that reverse the effect of common p53 cancer mutations. EMBO J 17: 1847–1859.
Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR et al. (1997). Thermodynamic stability of wild-type and mutant p53 core domain. Proc Natl Acad Sci USA 94: 14338–14342.
Bullock AN, Henckel J, Fersht AR . (2000). Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene 19: 1245–1256.
Butler JS, Loh SN . (2003). Structure, function, and aggregation of the zinc-free form of the p53 DNA binding domain. Biochemistry 42: 2396–2403.
Buzek J, Latonen L, Kurki S, Peltonen K, Laiho M . (2002). Redox state of tumor suppressor p53 regulates its sequence-specific DNA binding in DNA-damaged cells by cysteine 277. Nucleic Acids Res 30: 2340–2348.
Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P et al. (2002a). Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8: 282–288.
Bykov VJN, Issaeva N, Selivanova G, Wiman KG . (2002b). Mutant p53-dependent growth suppression distinguishes PRIMA-1 from known anticancer drugs: a statistical analysis of information in the National Cancer Institute database. Carcinogenesis 23: 2011–2018.
Bykov VJN, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J et al. (2005b). Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem 280: 30384–30391.
Bykov VJN, Selivanova G, Wiman KG . (2003). Small molecules that reactivate mutant p53. Eur J Cancer 39: 1828–1834.
Bykov VJN, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, Wiman KG . (2005a). PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene 24: 3484–3491.
Canadillas JM, Tidow H, Freund SM, Rutherford TJ, Ang HC, Fersht AR . (2006). Solution structure of p53 core domain: structural basis for its instability. Proc Natl Acad Sci USA 103: 2109–2114.
Caron de Fromentel C, Gruel N, Venot C, Debussche L, Conseiller E, Dureuil C et al. (1999). Restoration of transcriptional activity of p53 mutants in human tumour cells by intracellular expression of anti-p53 single chain Fv fragments. Oncogene 18: 551–557.
Chene P . (2003). Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer 3: 102–109.
Chipuk JE, Maurer U, Green DR, Schuler M . (2003). Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell 4: 371–381.
Christophorou MA, Martin-Zanca D, Soucek L, Lawlor ER, Brown-Swigart L, Verschuren EW et al. (2005). Temporal dissection of p53 function in vitro and in vivo. Nat Genet 37: 718–726.
Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI . (2006). The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443: 214–217.
Cho Y, Gorina S, Jeffrey PD, Pavletich NP . (1994). Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265: 346–355.
Cohen PA, Hupp TR, Lane DP, Daniels DA . (1999). Biochemical characterization of different conformational states of the Sf9 cell-purified p53His175 mutant protein. FEBS Lett 463: 179–184.
Cook A, Milner J . (1990). Evidence for allosteric variants of wild-type p53, a tumour suppressor protein. Br J Cancer 61: 548–552.
Di Como CJ, Prives C . (1998). Human tumor-derived p53 proteins exhibit binding site selectivity and temperature sensitivity for transactivation in a yeast-based assay. Oncogene 16: 2527–2539.
Espinosa JM, Emerson BM . (2001). Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell 8: 57–69.
Fields S, Jang SK . (1990). Presence of a potent transcription activating sequence in the p53 protein. Science 249: 1046–1049.
Foster BA, Coffey HA, Morin MJ, Rastinejad F . (1999). Pharmacological rescue of mutant p53 conformation and function. Science 286: 2507–2510.
Friedler A, Hansson LO, Veprintsev DB, Freud SM, Rippin TM, Nikolova PV et al. (2002). A peptide that binds and stabilizes p53 core domain: chaperone strategy for rescue of oncogenic mutants. Proc Natl Acad Sci USA 99: 937–942.
Friedler A, Veprintsev DB, Hansson LO, Fersht, AR . (2003). Kinetic instability of p53 core domain mutants: implications for rescue by small molecules. J Biol Chem 278: 24108–24112.
Friedlander P, Legros Y, Soussi T, Prives C . (1996). Regulation of mutant p53 temperature-sensitive DNA binding. J Biol Chem 271: 25468–25478.
Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C . (2001). A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol 21: 1874–1887.
Gannon JV, Greaves R, Iggo R, Lane DP . (1990). Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J 9: 1595–1602.
Gorina S, Pavletich NP . (1996). Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science 274: 1001–1005.
Halazonetis TD, Kandil AN . (1993). Conformational shifts propagate from the oligomerization domain of p53 to its tetrameric DNA binding domain and restore DNA binding to select p53 mutants. EMBO J 12: 5057–5064.
Hansen S, Hupp TR, Lane DP . (1996). Allosteric regulation of the thermostability and DNA binding activity of human p53 by specific interacting proteins. J Biol Chem 271: 3917–3924.
Hupp TR, Lane DP . (1994). Allosteric activation of latent p53 tetramers. Curr Biol 4: 865–875.
Hupp TR, Meek DW, Midgley CA, Lane DP . (1993). Activation of the cryptic DNA binding function of mutant forms of p53. Nucleic Acids Res 21: 3167–3174.
Issaeva N, Friedler A, Bozko P, Wiman KG, Fersht AR, Selivanova G . (2003). Rescue of mutants of the tumor suppressor p53 in cancer cells by a designed peptide. Proc Natl Acad Sci USA 100: 13303–13307.
Joerger AC, Allen MD, Fersht AR . (2004). Crystal structure of a superstable mutant of human p53 core domain. Insights into the mechanism of rescuing oncogenic mutations. J Biol Chem 279: 1291–1296.
Joerger AC, Ang HC, Fersht AR . (2006). Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc Natl Acad Sci USA 103: 15056–15061.
Joerger AC, Ang HC, Veprintsev DB, Blair CM, Fersht AR . (2005a). Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J Biol Chem 280: 16030–16037.
Joerger AC, Friedler A, Fersht AR . (2005b). Wild type p53 conformation, structural consequences of p53 mutations and mechanisms of mutant p53 rescue. In: Hainaut P, Wiman KG (Eds). 25 years of p53 Research pp 377–397.
Kim AL, Raffo AJ, Brandt-Rauf PW, Pincus MR, Monaco R, Abarzua P et al. (1999). Conformational and molecular basis for induction of apoptosis by a p53 C-terminal peptide in human cancer cells. J Biol Chem 274: 34924–34931.
Lane DP, Lain S . (2002). Therapeutic exploitation of the p53 pathway. Trends Mol Med 8: S38–S42.
Li Y, Mao Y, Brandt-Rauf PW, Williams AC, Fine RL . (2005). Selective induction of apoptosis in mutant p53 premalignant and malignant cancer cells by PRIMA-1 through the c-Jun-NH2-kinase pathway. Mol Cancer Ther 4: 901–909.
Liu WL, Midgley C, Stephen C, Saville M, Lane DP . (2001). Biological significance of a small highly conserved region in the N terminus of the p53 tumour suppressor protein. J Mol Biol 313: 711–731.
Lu X, Lane DP . (1993). Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell 75: 765–778.
Luu Y, Bush J, Cheung Jr KJ, Li G . (2002). The p53 stabilizing compound CP-31398 induces apoptosis by activating the intrinsic Bax/mitochondrial/caspase-9 pathway. Exp Cell Res 276: 214–222.
Marin MC, Jost CA, Brooks LA, Irwin MS, O’Nions J, Tidy JA et al. (2000). A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet 25: 47–54.
McCormick F . (2001). Cancer gene therapy: fringe or cutting edge? Nat Rev Cancer 1: 130–141.
McKinney K, Mattia M, Gottfredi V, Prives C . (2004). p53 linear diffusion along DNA requires its C terminus. Mol Cell 16: 413–424.
McKinney K, Prives C . (2002). Efficient specific DNA binding by p53 requires both its central and C-terminal domains as revealed by studies with high-mobility group 1 protein. Mol Cell Biol 22: 6797–6808.
Midgley CA, Desterro JM, Saville MK, Howard S, Sparks A, Hay RT et al. (2000). An N-terminal p14ARF peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 19: 2312–2323.
Milner J, Watson JV . (1990). Addition of fresh medium induces cell cycle and conformation changes in p53, a tumour suppressor protein. Oncogene 5: 1683–1690.
Müller-Tiemann BF, Halazonetis TD, Elting JJ . (1998). Identification of an additional negative regulatory region for p53 sequence-specific DNA binding. Proc Natl Acad Sci USA 95: 6079–6084.
Nahi H, Lehmann S, Mollgard L, Bengtzen S, Selivanova G, Wiman KG et al. (2004). Effects of PRIMA-1 on chronic lymphocytic leukaemia cells with and without hemizygous p53 deletion. Br J Hematol 127: 285–291.
Nevins JR . (2001). The Rb/E2F pathway and cancer. Hum Mol Genet 10: 699–703.
Nichols NM, Matthews KS . (2002). Human p53 phosphorylation mimic, S392E, increases nonspecific DNA affinity and thermal stability. Biochemistry 41: 170–178.
Niewolik D, Vojtesek B, Kovarik J . (1995). p53 derived from human tumour cell lines and containing distinct point mutations can be activated to bind its consensus target sequence. Oncogene 10: 881–890.
Nikolova PV, Wong KB, DeDecker B, Henckel J, Fersht AR . (2000). Mechanism of rescue of common p53 cancer mutations by second-site suppressor mutations. EMBO J 19: 370–378.
North S, Pluquet O, Maurici D, El-Ghissassi F, Hainaut P . (2002). Restoration of wild-type conformation and activity of a temperature-sensitive mutant of p53 (p53(V272M)) by the cytoprotective aminothiol WR1065 in the esophageal cancer cell line TE-1. Mol Carcinog 33: 181–188.
Okorokov AL, Sherman MB, Plisson C, Grinkevich V, Sigmudsson K, Selivanova G et al. (2006). The structure of p53 tumour suppressor protein reveals the basis for its functional plasticity. EMBO J 25: 5191–5200.
Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P . (2002). The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat 19: 607–614.
Ory K, Legros Y, Auguin C, Soussi T . (1994). Analysis of the most representative tumour-derived p53 mutants reveals that changes in protein conformation are not correlated with loss of transactivation or inhibition of cell proliferation. EMBO J 13: 3496–3504.
Peng Y, Chen L, Li C, Lu W, Chen J . (2001). Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization. J Biol Chem 276: 40583–40590.
Pluquet O, North S, Richard MJ, Hainaut P . (2003). Activation of p53 by the cytoprotective aminothiol WR1065: DNA-damage-independent pathway and redox-dependent modulation of p53 DNA-binding activity. Biochem Pharmacol 65: 1129–1137.
Rehman A, Chahal MS, Tang X, Bruce JE, Pommier Y, Daoud SS . (2005). Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells. Breast Cancer Res 7: R765–R774.
Rippin TM, Bykov VJ, Freund SM, Selivanova G, Wiman KG, Fersht AR . (2002). Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene 21: 2119–2129.
Rökaeus N, Klein G, Wiman KG, Szekely L, Mattsson K . (2006). PRIMA-1(MET) induces nucleolar accumulation of mutant p53 and PML nuclear body-associated proteins. Oncogene Epub online.
Roth J, Koch P, Contente A, Dobbelstein M . (2000). Tumor-derived mutations within the DNA-binding domain of p53 that phenotypically resemble the deletion of the proline-rich domain. Oncogene 19: 1834–1842.
Rudiger S, Freund SM, Veprintsev DB, Fersht AR . (2002). CRINEPT-TROSY NMR reveals p53 core domain bound in an unfolded form to the chaperone Hsp90. Proc Natl Acad Sci USA 99: 11085–11090.
Sabapathy K, Klemm M, Jaenisch R, Wagner EF . (1997). Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J 16: 6217–6229.
Samuels-Lev Y, O’Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S et al. (2001). ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8: 781–794.
Selivanova G . (2001). Mutant p53: the loaded gun. Curr Opin Invest Drugs 2: 1136–1141.
Selivanova G, Iotsova V, Okan I, Fritsche M, Strom M, Groner B et al. (1997). Restoration of the growth suppression function of mutant p53 by a synthetic peptide derived from the p53 C-terminal domain. Nat Med 3: 632–638.
Selivanova G, Kawasaki T, Ryabchenko L, Wiman KG . (1998). Reactivation of mutant p53: a new strategy for cancer therapy. Semin Cancer Biol 8: 369–378.
Selivanova G, Ryabchenko L, Jansson E, Iotsova V, Wiman KG . (1999). Reactivation of mutant p53 through interaction of a C-terminal peptide with the core domain. Mol Cell Biol 19: 3395–3402.
Selivanova G, Wiman KG . (2001). Functional rescue of mutant p53 as a strategy to combat cancer. In: Maruta H (ed). Tumor-suppressing viruses, genes and drugs. Academic Press: San Diego, CA, USA, pp 397–415.
Seo YR, Kelley MR, Smith ML . (2002). Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci USA 99: 14548–14553.
Sharp S, Workman P . (2006). Inhibitors of the HSP90 molecular chaperone: current status. 95: 323–348.
Shiraishi K, Kato S, Han SY, Liu W, Otsuka K, Sakayori M et al. (2004). Isolation of temperature-sensitive p53 mutations from a comprehensive missense mutation library. J Biol Chem 279: 348–355.
Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD et al. (2006). The consensus coding sequences of human breast and colorectal cancers. Science 314: 268–274.
Snyder EL, Meade BR, Saenz CC, Dowdy SF . (2004). Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol 2: E36.
Strano S, Munarizz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M et al. (2000). Physical and functional interaction between p53 mutants and different isoforms of p73. J Biol Chem 275: 29503–29512.
Takimoto R, Wang W, Dicker DT, Rastinejad F, Lyssikatos J, El-Deiry WS . (2002). The mutant p53-conformation modifying drug, CP-31398, can induce apoptosis of human cancer cells and can stabilize wild-type p53 protein. Cancer Biol Ther 1: 47–55.
Vives V, Slee E, Lu X . (2006). ASPP2: a gene that controls life and death in vivo. Cell Cycle 5: 2187–2190.
Vojtesek B, Dolezalova H, Lauerova L, Svitakova M, Havlis P, Kovarik J et al. (1995). Conformational changes in p53 analysed using new antibodies to the core DNA binding domain of the protein. Oncogene 10: 389–393.
Vousden K, Lu X . (2002). Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604.
Walerych D, Kudla G, Gutkowska M, Wawrzynow B, Muller L, King FW et al. (2004). Hsp90 chaperones wild-type p53 tumor suppressor protein. J Biol Chem 279: 48836–48845.
Wang PL, Sait F, Winter G . (2001). The ‘wildtype’ conformation of p53: epitope mapping using hybrid proteins. Oncogene 20: 2318–2324.
Wang W, Kim S-H, El-Deiry WS . (2006). Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci USA 103: 11003–11008.
Wang W, Takimoto R, Rastinejad F, El-Deiry WS . (2003). Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding. Mol Cell Biol 23: 2171–2181.
Wieczorek AM, Waterman JL, Waterman MJ, Halazonetis TD . (1996). Structure-based rescue of common tumor-derived p53 mutants. Nat Med 2: 1143–1146.
Wiman KG . (2006). Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ 13: 921–926.
Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M . (2003). CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene 22: 8233–8245.
Wong KB, DeDecker BS, Freund SM, Proctor MR, Bycroft M, Fersht AR . (1999). Hot-spot mutants of p53 core domain evince characteristic local structural changes. Proc Natl Acad Sci USA 96: 8438–8442.
Zhang W, Guo XY, Hu GY, Liu WB, Shay JW, Deisseroth AB . (1994). A temperature-sensitive mutant of human p53. EMBO J 13: 2535–2544.
Zheleva DI, Lane DP, Fischer PM . (2003). The p53-Mdm2 pathway: targets for the development of new anticancer therapeutics. Mini Rev Med Chem 3: 257–270.
Acknowledgements
We thank the Swedish Cancer Society, EU FP6, the Cancer Society of Stockholm, the Swedish Research Council and the Karolinska Institutet for generous support, and Andrej Okorokov for help with Figure 2. We apologize to all our colleagues whose work could not be cited because of space limitations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selivanova, G., Wiman, K. Reactivation of mutant p53: molecular mechanisms and therapeutic potential. Oncogene 26, 2243–2254 (2007). https://doi.org/10.1038/sj.onc.1210295
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210295
Keywords
This article is cited by
-
Role of senescent tumor cells in building a cytokine shield in the tumor microenvironment: mathematical modeling
Journal of Mathematical Biology (2023)
-
DNA damage response revisited: the p53 family and its regulators provide endless cancer therapy opportunities
Experimental & Molecular Medicine (2022)
-
Protein mimetic amyloid inhibitor potently abrogates cancer-associated mutant p53 aggregation and restores tumor suppressor function
Nature Communications (2021)
-
Evidence for a non-stochastic two-field hypothesis for persistent skin cancer risk
Scientific Reports (2020)
-
Styrylcoumarin 7-SC2 induces apoptosis in SW480 human colon adenocarcinoma cells and inhibits azoxymethane-induced aberrant crypt foci formation in BALB/c mice
Medicinal Chemistry Research (2020)