Abstract
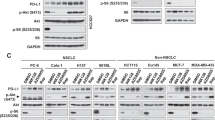
Rapamycin and several analogs, such as CCI-779 and RAD001, are currently undergoing clinical evaluation as anticancer agents. In this study, we show that inhibition of mammalian target of rapamycin (mTOR) signaling by rapamycin leads to an increase of Akt phosphorylation in Rh30 and RD human rhabdomyosarcoma cell lines and xenografts, and insulin-like growth factor (IGF)-II-treated C2C12 mouse myoblasts and IGF-II-overexpressing Chinese hamster ovary cells. RNA interference-mediated knockdown of S6K1 also results in an increase of Akt phosphorylation. These data suggest that mTOR/S6K1 inhibition either by rapamycin or small interfering RNA (siRNA) triggers a negative feedback loop, resulting in the activation of Akt signaling. We next sought to investigate the mechanism of this negative feedback regulation from mTOR to Akt. Suppression of insulin receptor substrate (IRS)-1 and tuberous sclerosis complex-1 by siRNAs failed to abrogate rapamycin-induced upregulation of Akt phosphorylation in both Rh30 and RD cells. However, pretreatment with h7C10 antibody directed against insulin-like growth factor-1 receptor (IGF-1R) led to a blockade of rapamycin-induced Akt activation. Combined mTOR and IGF-1R inhibition with rapamycin and h7C10 antibody, respectively, resulted in additive inhibition of cell growth and survival. These data suggest that rapamycin mediates Akt activation through an IGF-1R-dependent mechanism. Thus, combining an mTOR inhibitor and an IGF-1R antibody/inhibitor may be an appropriate strategy to enhance mTOR-targeted anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abraham RT, Wiederrecht GJ . (1996). Immunopharmacology of rapamycin. Annu Rev Immunol 14: 483–510.
Dagher R, Helman L . (1999). Rhabdomyosarcoma: an overview. Oncologist 4: 34–44.
Fingar DC, Blenis J . (2004). Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171.
Fingar DC, Salama S, Tsou C, Harlow E, Blenis J . (2002). Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487.
Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels PJ, Haeuw JF et al. (2005). A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer 113: 316–328.
Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS et al. (2002). Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem 277: 30958–30967.
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H et al. (2004). The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166: 213–223.
Hay N . (2005). The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8: 179–183.
Hay N, Sonenberg N . (2004). Upstream and downstream of mTOR. Genes Dev 18: 1926–1945.
Hidalgo M, Rowinsky EK . (2000). The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene 19: 6680–6686.
Huang S, Houghton PJ . (2003). Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol 3: 371–377.
Inoki K, Li Y, Zhu T, Wu J, Guan KL . (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657.
Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF et al. (2000). Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest 105: 199–205.
Lawlor MA, Alessi DR . (2001). PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910.
LeRoith D, Baserga R, Helman L, Roberts Jr CT . (1995). The role of the insulin-like growth factor-I receptor in cancer. Ann NY Acad Sci 766: 402–408.
LeRoith D, Helman L . (2004). The new kid on the block(ade) of the IGF-1 receptor. Cancer Cell 5: 201–202.
Manning BD . (2004). Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167: 399–403.
Merlino G, Helman L . (1999). Rhabdomyosarcoma – working out the pathways. Oncogene 18: 5340–5348.
Minniti CP, Luan D, O’Grady C, Rosenfeld RG, Oh Y, Helman LJ . (1995). Insulin-like growth factor II overexpression in myoblasts induces phenotypic changes typical of the malignant phenotype. Cell Growth Differ 6: 263–269.
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D et al. (2006). mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508.
Pappo AS, Shapiro DN, Crist WM, Maurer HM . (1995). Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol 13: 2123–2139.
Potter CJ, Pedraza LG, Xu T . (2002). Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4: 658–665.
Radimerski T, Montagne J, Hemmings-Mieszczak M, Thomas G . (2002). Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6 K signaling. Genes Dev 16: 2627–2632.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H et al. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101.
Schmelzle T, Hall MN . (2000). TOR, a central controller of cell growth. Cell 103: 253–262.
Shah OJ, Wang Z, Hunter T . (2004). Inappropriate activation of the TSC/Rheb/mTOR/S6 K cassette induces IRS1/2 depletion, insulin resistance and cell survival deficiencies. Curr Biol 14: 1650–1656.
Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P et al. (2004). Rheb is an essential regulator of S6 K in controlling cell growth in Drosophila. Nat Cell Biol 5: 559–565.
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H et al. (2005). Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052–7058.
Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA et al. (1991). Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352: 73–77.
Tremblay F, Marette A . (2001). Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 276: 38052–38060.
Wan X, Helman LJ . (2002). Effect of insulin-like growth factor II on protecting myoblast cells against cisplatin-induced apoptosis through p70 S6 kinase pathway. Neoplasia 4: 400–408.
Wan X, Helman LJ . (2003). Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene 22: 8205–8211.
Wan X, Mendoza A, Khanna C, Helman LJ . (2005). Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res 65: 2406–2411.
White MF . (1998). The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem 182: 3–11.
Womer RB, Pressey JG . (2000). Rhabdomyosarcoma and soft tissue sarcoma in childhood. Curr Opin Oncol 12: 337–344.
Zhang L, Kim M, Choi YH, Goemans B, Yeung C, Hu Z et al. (1999). Diminished G1 checkpoint after gamma-irradiation and altered cell cycle regulation by insulin-like growth factor II overexpression. J Biol Chem 274: 13118–13126.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Merck Inc. for providing us with the h7C10 antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, X., Harkavy, B., Shen, N. et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26, 1932–1940 (2007). https://doi.org/10.1038/sj.onc.1209990
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209990
Keywords
This article is cited by
-
NUAK1 coordinates growth factor-dependent activation of mTORC2 and Akt signaling
Cell & Bioscience (2023)
-
mTOR inhibition suppresses Myc-driven polyposis by inducing immunogenic cell death
Oncogene (2023)
-
MicroRNA based combinatorial therapy against TKIs resistant CML by inactivating the PI3K/Akt/mTOR pathway: a review
Medical Oncology (2023)
-
Oxadiazol-based mTOR inhibitors with potent antiproliferative activities: synthetic and computational modeling
Molecular Diversity (2022)
-
Therapeutically actionable signaling node to rescue AURKA driven loss of primary cilia in VHL-deficient cells
Scientific Reports (2021)