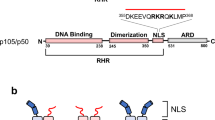
Abstract
Rel/NF-κB transcription factors form homo- and heterodimers with different DNA binding site specificities and DNA binding affinities. Several intracellular pathways evoked by a wide range of biological factors and environmental conditions can lead to the activation of Rel/NF-κB dimers by signaling degradation of the inhibitory IκB protein. In the nucleus Rel/NF-κB dimers modulate the expression of a variety of genes including those encoding cytokines, growth factors, acute phase response proteins, immunoreceptors, other transcription factors, cell adhesion molecules, viral proteins and regulators of apoptosis. The primary focus of this review is on structural and functional aspects of Rel/NF-κB:DNA complexes and their formation. The salient features of the Rel/NF-κB dimer:DNA structure are described, as are modes of transcriptional regulation by phosphorylation, altered DNA binding properties, varying protein conformations, and interactions with IκB proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baeuerle PA and Henkel T. . 1994 Annu. Rev. Immunol. 12: 141–179.
Baldwin Jr AS. . 1996 Annu. Rev. Immunol. 14: 649–683.
Batchelor AH, Piper DE, de la Brousse FC, McKnight SL and Wolberger C. . 1998 Science 279: 1037–1041.
Chen FE, Huang D-B, Chen Y-Q and Ghosh G. . 1998a Nature 391: 410–413.
Chen Y-Q, Ghosh S and Ghosh G. . 1998b Nature Struc. Biol. 5: 67–73.
Coleman TA, Kunsch C, Maher M, Ruben SM and Rosen CA. . 1993 Mol. Cell. Biol. 13: 3850–3859.
Cramer P, Larson CJ, Verdine GL and Müller CW. . 1997 EMBO J. 16: 7078–7090.
Fujita T, Nolan GP, Ghosh S and Baltimore D. . 1992 Genes Dev. 6: 775–787.
Ganchi PA, Sun SC, Greene WC and Ballard DW. . 1993 Mol. Cell. Biol. 13: 7826–7835.
Ghosh G, van Duyne G, Ghosh S and Sigler PB. . 1995 Nature 373: 303–310.
Ghosh S, May MJ and Kopp EB. . 1998 Ann. Rev. Immunol. 16: 225–260.
Gilmore TD. . 1999 Oncogene 18: 6842–6844.
Gorina S and Pavletich NP. . 1996 Science 274: 1001–1005.
Hayashi T, Sekine T and Okamoto T. . 1993 J. Biol. Chem. 268: 26790–26795.
Hrdlicková R, Nehyba J and Bose Jr HR. . 1995 J. Virol. 69: 3369–3380.
Huang D-B, Huxford T, Chen Y-Q and Ghosh G. . 1997 Structure 5: 1427–1436.
Huxford T, Huang DB, Malek S and Ghosh G. . 1998 Cell 95: 759–770.
Jacobs MD and Harrison SC. . 1998 Cell 95: 749–758.
Karin M. . 1999 Oncogene 18: 6867–6874.
Kunsch C, Ruben SM and Rosen CA. . 1992 Mol. Cell. Biol. 12: 4412–4421.
Kushner DB and Ricciardi RP. . 1999 Mol. Cell. Biol. 19: 2169–2179.
Li CC, Dai RM, Chen E and Longo DL. . 1994a J. Biol. Chem. 269: 30089–30092.
Li CC, Korner M, Ferris DK, Chen E, Dai RM and Longo DL. . 1994b Biochem. J. 303: 499–506.
McElhinny JA, Trushin SA, Bren GD, Chester N and Paya CV. . 1996 Mol. Cell. Biol. 16: 899–906.
Müller CW, Rey FA, Sodeoka M, Verdine GL and Harrison SC. . 1995 Nature 373: 311–317.
Nabel G and Baltimore D. . 1987 Nature 326: 711–713.
Nehyba J, Hrdlicková R and Bose Jr HR. . 1997 Oncogene 14: 2881–2897.
Pahl HL. . 1999 Oncogene 18: 6853–6866.
Rottjakob EM, Sachdev S, Leanna CA, McKinsey TA and Hannink M. . 1996 J. Virol. 70: 3176–3188.
Schreck R, Zorbas H, Winnacker E and Baeuerle PA. . 1990 Nucleic Acids Res. 18: 6497–6502.
Sen R and Baltimore D. . 1986 Cell 47: 921–928.
Sengchanthalangsy LL, Datta S, Huang D-B, Anderson E, Braswell EH and Ghosh G. . 1999 J. Mol. Biol. 289: 1029–1040.
Siebenlist U, Franzoso G and Brown K. . 1994 Annu. Rev. Cell. Biol. 10: 405–455.
Tisne C, Hantz E, Hartmann B and Delepierre M. . 1998 J. Mol. Biol. 279: 127–142.
Tisne C, Hartmann B and Delepierre M. . 1999 Biochemistry 38: 3883–3894.
Toledano MB, Ghosh D, Trinh F and Leonard WJ. . 1993 Mol. Cell. Biol. 13: 852–860.
Zabel U, Schreck R and Baeuerle PA. . 1991 J. Biol. Chem. 266: 252–260.
Zhong H, Voll RE and Ghosh S. . 1998 Mol. Cell 1: 661–671.
Acknowledgements
The authors thank AK Aggarwal for generously providing the coordinates of the NF-κB:IFN κB DNA complex. Thanks are also extended to T Huxford for the figure of IκBα. This work was supported by fellowships from the NSF and the Lucille P Markey Charitable Trust to F Chen. This work was also supported by a NCI research grant (CA71871) to G Ghosh.
Author information
Authors and Affiliations
Rights and permissions
About this article
This article is cited by
-
NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype
Cell Communication and Signaling (2023)
-
High throughput screen for the improvement of inducible promoters for tumor microenvironment cues
Scientific Reports (2022)
-
C11orf95-RELA fusion drives aberrant gene expression through the unique epigenetic regulation for ependymoma formation
Acta Neuropathologica Communications (2021)
-
Nuclear KIT induces a NFKBIB-RELA-KIT autoregulatory loop in imatinib-resistant gastrointestinal stromal tumors
Oncogene (2019)
-
HBx-induced MiR-1269b in NF-κB dependent manner upregulates cell division cycle 40 homolog (CDC40) to promote proliferation and migration in hepatoma cells
Journal of Translational Medicine (2016)