Abstract
Prefrontal cortical dopamine (DA) regulates various executive cognitive functions, including attention and working memory. Efforts to enhance prefrontal-related cognition, which have focused on catecholaminergic stimulant drugs, have been unsatisfactory. Recently, the demonstration that a functional polymorphism in the catecholamine-O-methyltransferase (COMT) gene impacts prefrontal cognition raises the possibility of a novel pharmacological approach for the treatment of prefrontal lobe executive dysfunction. To explore in a proof of concept study the effects of tolcapone, a CNS penetrant specific COMT inhibitor, we performed a randomized, double blind, placebo controlled, and crossover design of this drug in normal subjects stratified by COMT (val158met) genotype. COMT enzyme activity was determined in peripheral blood. Forty-seven normal volunteers with no family history of psychiatric disorders underwent neuropsychological testing and 34 of those subjects underwent physiological measurement of prefrontal information processing assessed by blood oxygen level-dependent functional magnetic resonance imaging (fMRI). We found significant drug effects on measures of executive function and verbal episodic memory and a significant drug by genotype interaction on the latter, such that individuals with val/val genotypes improved, whereas individuals with met/met genotypes worsened on tolcapone. fMRI revealed a significant tolcapone-induced improvement in the efficiency of information processing in prefrontal cortex during a working memory test. This study demonstrates enhancement of prefrontal cortical function in normal human subjects with a nonstimulant drug having COMT inhibitory activity. Our results are consistent with data from animal studies and from computational models of the effects of selective enhancement of DA signaling in the prefrontal cortex.
Similar content being viewed by others
INTRODUCTION
Recent studies have shown that a polymorphism in the catecholamine-O-methyltransferase (COMT) gene (Val108/158 Met) that encodes enzyme variants with different activity can predict prefrontal cortical function presumably because of varying the availability of synaptic dopamine (DA). This genetic effect has been demonstrated in humans for performance on a variety of cognitive tests, such as working memory and executive function (Egan et al, 2001; Ho et al, 2005) as well as prefrontal physiology as assessed by functional magnetic resonance imaging (fMRI) (Egan et al, 2001; Mattay et al, 2003). There are also reports that COMT genotype affects function on declarative memory tests referable to processing in temporal lobe structures implicated in long-term memory (de Frias et al, 2004)
Converging evidence from electrophysiological studies in experimental animals indicates that DA focuses and stabilizes prefrontal cortical networks by modulating NMDA, non-NMDA, and GABAergic currents (Seamans and Yang, 2004). Consistent with this basic evidence, neuroimaging studies with pharmacological manipulations in normal humans using indirect agonists such as amphetamine or with levodopa treatment in patients with Parkinson's disease have demonstrated that increasing DA activity, to some extent, ‘focuses’ PFC activity during executive cognition (ie it enhances prefrontal physiologic ‘efficiency’) (Cools et al, 2002; Mattay et al, 2000, 2002, 2003). In contrast to psychostimulant drugs (eg amphetamine, methylphenidate), which target all biogenic amines throughout the brain, animal studies showed that COMT appears to specifically affect extracellular DA levels, primarily in cortical regions where DA transporters are functionally inconsequential (Gogos et al, 1998; Mazei et al, 2002; Moron et al, 2002; Tunbridge et al, 2004).
A distinct but more specific pharmacological approach to regulate cortical DA signaling may be achieved by increasing extracellular DA through inhibition of COMT. In humans, COMT is found in high concentrations in the cortex relative to subcortical regions such as the striatum and amygdala (Lloyd et al, 1975; Matsumoto et al, 2003) consistent with the absence of functional DA transporters in cortical areas such as prefrontal cortex and hippocampus (Hall et al, 1999; Mazei et al, 2002; Pozzi et al, 1994; Sesack et al, 1998). Thus, COMT appears to represent the main catabolic pathway by which DA is removed from the cortical synaptic cleft (Karoum et al, 1994). Even though the knockout model presents a complex neurochemical neural dynamics that goes beyond the mere synaptic neurotransmitter release or metabolism, studies of COMT knock-out mice and of COMT inhibitors in rodents indicate that COMT has little impact on norepinephrine flux in prefrontal cortex, perhaps because of the abundance of functional norepinephrine transporters (Gogos et al, 1998; Tunbridge et al, 2004). Thus, relatively specific manipulation of prefrontal DA through blockade of the activity of COMT may represent a novel approach to improve cognitive function by increasing DA-mediated prefrontal signal-to-noise ratio, a measure of response strength relative to background noise. Moreover, because COMT does not appear to significantly impact extracellular DA levels in the rat striatum (Gogos et al, 1998; Li et al, 1998; Tunbridge et al, 2004) it might be expected that these effects would occur without psychostimulant properties and abuse potential.
In the last 30 years, a number of compounds with inhibitory activity on COMT were discovered (Guldberg and Marsden, 1975). Among them, tolcapone (Zurcher et al, 1990a, 1990b) proved to be the most potent in vitro. Whereas most of these COMT inhibitors inhibit peripheral COMT with greater potency than the enzyme present in the central nervous system, tolcapone does penetrate the blood–brain barrier (Ceravolo et al, 2002) and inhibits brain COMT activity in vivo with an inhibitory dose 50 (ID50) value of 26–28 mg/kg in the rodent (Da Prada et al, 1991; Zurcher et al, 1991). In behavioral studies in rats, intraperitoneal administration of tolcapone following pretraining facilitated spatial working memory. Similarly, tolcapone improved the performance of senescent poor-performing rats in a spatial memory task (Liljequist et al, 1997). In patients with Parkinson's disease, tolcapone was reported to improve function in attentional tasks, auditory verbal short-term memory, visuo-spatial recall, and constructional praxia, changes that are not typically associated with levodopa therapy alone (Gasparini et al, 1997). Taken together, these data suggest that tolcapone may improve some aspects of cognition. To our knowledge, this possibility has not been tested in normal human subjects.
In this study, we have evaluated the effects of tolcapone on measures of cognitive function and prefrontal cortical information processing in normal subjects. Specifically, based on the prior evidence of the effects of genetic variation in COMT on cognition and prefrontal cortical physiology, we predicted that COMT inhibition in normal volunteers would enhance prefrontally mediated cognitive function as measured on specific cognitive tests and the efficiency of this processing assayed with fMRI. We also predicted that because COMT Val108/158Met genotype influences performance on cognitive tests of prefrontal cortex function such as working memory and executive function (Egan et al, 2001; Goldberg et al, 2003) as well as prefrontal efficiency as assessed by fMRI (Egan et al, 2001; Mattay et al, 2002, 2003), that genotype would interact with the effects of tolcapone, such that individuals with val alleles, because of their higher enzyme activity and putatively lower DA concentrations in the DLPC, would show greater benefit.
METHODS
Subjects and Treatment
Participants
Normal volunteers were recruited from local and national sources as volunteers for the ‘CBDB/NIMH Sibling Study’. Briefly, all participants gave written informed consent to be part of an institutional review board-approved protocol. All participants underwent a structured clinical interview (SCID-I) to rule out an active Axis I diagnosis and a questionnaire for evaluation of personality disorder to rule out Axis II diagnosis. Diagnosis was made following DSM-IV criteria. The family history was obtained by a trained research nurse using a Sib Study Genogram that gathers information up to third degree relatives and beyond. The sample met the Hardy–Weinberg predictions (Hardy, 1908; Weinberg, 1908). Participants with a family history of schizophrenia, schizoaffective disorder, or schizophrenia spectrum disorder (schizotypal, paranoid, schizoid personality, or delusional disorder) were excluded from the study. Participants were aged 18 to 55 years and had an IQ greater than 90. Normal volunteers taking any type of medication on a regular basis were excluded form the study. To take part in the study, normal volunteers needed to be off all medications for 6 weeks. Individuals with significant medical conditions that might affect the central nervous or cardiovascular system, or liver function, a history of head trauma with loss of consciousness for longer than 5 min, or other drug or alcohol abuse within the past 3 months were excluded. Because of the potential toxic effect of tolcapone on liver function, subjects with a history of alcohol dependence were excluded. Demographics of the sample are listed in Table 1. Forty-seven individuals participated in the study. Seventy-eight percent of the participants were Caucasians of European ancestry, 11% were African American, 7% were Asians, 2% were Hispanics, and 2% mixed race. Demographic breakdown across the three genotypes groups did not differ (Table 1).
Treatment
Subjects were enrolled as outpatients for a double-blind, placebo controlled, and crossover study lasting 3 weeks. A table of random numbers was used to prepare the randomization. Both tolcapone and placebo were coded. Coded tolcapone was administered for 7 days at a dose of 100 mg three times a day on the first day and then at a dose of 200 mg three times a day for the next 6 days. Neuropsychological testing and fMRI were performed on the seventh day. After a one-week wash-out period following the first arm, the subjects who received coded tolcapone received coded placebo three times a day and those who started on the coded placebo received coded tolcapone three times a day, in both cases for 7 days. At the end of both arms, coded compounds were discontinued over a 3-day period to avoid potential withdrawal reactions. To mask the urine discoloration produced by tolcapone, participants took also a coded capsule containing 100 mg of riboflavin twice daily during both arms of the protocol.
Test schedule
Prior studies have shown that plasma levels peak about 1.5–2.0 h after administration of tolcapone and maximum inhibition of COMT activity in red blood cells is achieved between 1 and 4 h following administration of the drug (Jorga et al, 1999; Jorga, 1998; Mannisto and Kaakkola, 1999). Therefore, neuropsychological testing (NPT) and fMRI were performed 2 h after the dose of tolcapone on day 7 and the neuropsychological battery was completed in 75–80 min for the NPT and 60–75 min for the fMRI. Functional MRI was started at 2 h after the morning dose and NPT was started at 2 h after the afternoon dose.
Neuropsychological Testing
The following tests were administered to all subjects: (1) N Back test of working memory and updating. We used a block design in which subjects perform first a block of 1 backs, then a block of 3 backs, a block of 0 backs, and finally a block of 2 backs. The dependent measure included accuracy and reaction time (RT) for the 0 Back, 1 Back, 2 Back and 3 Back conditions (this task was administered to 42 subjects); (2) verbal fluency (for categories), in which subjects are asked to generate as many words as they can in 1 min that fit within an exemplar category (eg animals, groceries); (3) verbal episodic memory (a list of 15 semantically unrelated words was presented orally by the examiner, followed by immediate free recall; the list was repeated three times); (4) continuous performance test of target detection and attention; (5) CANTAB intradimensional/extradimensional (ID/ED) componential test of set shifting; (6) letter number span; (7) trail Making B Test of attention, visual scanning, and rapid set switching; and (8) Wisconsin card sorting test of executive function.
These specific tests were selected because they have demonstrated sensitivity to COMT genotype (notably, N Back, CPT, verbal memory, ID/ED), engage prefrontal cortical processes, and/or demonstrate a response to psychostimulant medication (notably verbal fluency, trail making and letter number span) (D’Esposito et al, 2000; Gold et al, 1997; Gourovitch et al, 2000).
Profile of mood states
The POMS (McNair and Lorr, 1981), a self-rated questionnaire consisting of 65 items, was administered in order to assess whether tolcapone induced changes in mood or activation.
Data analysis
All cognitive variables were analyzed by repeated measure ANOVA in which drug status (on tolcapone, off tolcapone) served as the within-subject variable, and order (drug then placebo or placebo then drug) and COMT genotype (val homozygote, met homozygote, val/met heterozygote) served as between-subject class variables. Based on the earlier studies cited above, we were particularly interested in the main effects of drug and drug × genotype interactions, suggesting genotypic modulation of drug responses.
fMRI Data Acquisition
Each subject was scanned using a GE Sigma 3T scanner with a real-time functional imaging upgrade (Milwaukee, WI). BOLD fMRI (gradient echo EPI sequence, 24 axial 6 mm thick interleaved slices, TR/TE=2000/30 ms, flip angle=90°, FOV=24 cm, and matrix=64 × 64) was conducted while subjects performed three levels of the WM task. Stimuli were presented via a fiber-optic goggle system and the responses were recorded via a fiber-optic response box. During each treatment condition, four cycles of the WM task (1-Back, 2-Back, or 3-Back) alternating with the 0-Back (sensorimotor task) were administered. Each task combination was obtained in 4 min and 8 s, 124 whole-brain images with four cycles of 30 images each (15 during working memory (1-Back, 2-Back, or 3-Back) and 15 during the 0-Back task. The first four scans at the beginning of each time series were acquired to allow the signal to reach a steadystate and were not included in the final analysis. The order of the task combinations was counterbalanced across subjects but maintained within subjects across drug conditions. We used a block design for the fMRI task in which 1-, 2-, or 3-Back alternate with the 0-Back and were administered as separate runs. The procedure is precisely as described in a study of the effects of amphetamine on this task in normal subjects (Mattay et al, 2003). To preclude the effects of learning on brain activation patterns, all subjects were trained on the N-Back task until they reached a ceiling in their performance. The training was ‘subject specific’. This was performed during a separate session before the fMRI sessions. With such a design, although it is unlikely for individuals to improve on their N-Back performance scores during the fMRI procedure, there could be an improvement in cortical efficiency (ie a decrease in BOLD activity for the same level of performance).
Image Processing
In three subjects, imaging data could not be acquired at all three levels of the N-Back working memory task owing to time constraints, and in another three subjects, imaging data from one of the two sessions were lost owing to a computer malfunction. Image analysis, therefore, was limited to data from the 41 subjects with complete data sets. Whole-brain analysis was completed using SPM99 (http://www.fil.ion.ucl.ac.uk/spm), again as described elsewhere (Mattay et al, 2003). Briefly, images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 10 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Data sets were also screened for high quality (scan stability) as demonstrated by small motion correction (<5 mm translation and 3° rotation) and matched voxel variance across the drug and placebo sessions. Data from 34 subjects (8 met/met, 16 val/met, 10 val/val) met these stringent criteria and final image analysis was limited to the data from these subjects. Predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for the contrast of the working memory task (1-Back, 2-Back, or 3-Back) vs the sensorimotor control (0-Back) for each subject for each drug condition. These individual contrast images were then used in a conservative second-level random effects model that account for both scan-to-scan and subject-to-subject variability.
Because of our strong a priori hypothesis as noted above, and our use of a rigorous random effects statistical model, a statistical threshold of p<0.05, with a small volume correction (SVC—using 30 mm diameter spheres over the activation peaks in the right and left DLPFC) for multiple comparisons, was used to identify significant responses for all comparisons.
For the analysis, a series of one-tailed t-tests were performed to evaluate the main effects of the working memory task at each level of task difficulty during each drug condition. Analysis of variance (ANOVA) was then performed to explore drug, genotype, and working memory load effects. The latter analysis was constrained by using a mask obtained by combining the one-tailed t-test activation maps for each working memory task level during both placebo and tolcapone conditions. Post hoc analysis was performed using a region of interest approach. Volumes of interest (VOI) were defined in the right and left DLPFC (BA 9/46) encompassing voxels that showed a significant main effect of drug (p<0.02, in right DLPFC and p<0.03, SVC in left DLPFC). Using MARSBAR (http://www.marsbar.sourceforge.net), mean percentage change in BOLD signal was extracted from these VOI for each WM task state and each drug condition in every subject.
COMT Genotyping
Blood samples were collected from all participants, and DNA was extracted using standard methods. COMT val158met (rs4680 G to A) genotyping was determined using Taqman 5′-exonuclease allelic discrimination assay (Applied Biosystems, Foster City, CA) as described elsewhere (Chen et al, 2004).
COMT Enzyme Assay
COMT enzyme activity was determined in whole peripheral blood collected after 7 days of treatment with either placebo or tolcapone. Blood was obtained before and 4 h after the morning administration of placebo or tolcapone. The COMT enzyme activity assay is based on the organic solvent extraction method that separates the radioactive product, the methylated catechol, and the free radioactive co-enzyme, 3H-S-adenosyl-methionine (SAM). One hundred microgram of supernatant at a concentration of 5 μg protein/μl was transferred to a fresh microcentrifuge tube from each sample and equilibrated to temperature shortly before the enzyme assay. Five hundred microgram of the substrate mixture containing 10 mM Tris, pH 7.4, 1 mM MgCl2, 1.5 μCi of 3SAM, 10 μM of catechol, and 1 μM of DTT was added to each tube. The tubes were then incubated at 37°C for 20 min. The reactions were immediately terminated by placing the tubes on ice and adding 500 μl of 1 M HCl. The radioisotope-labeled catechol products from the reactions were extracted and determined by mixing the reaction mixture with 10 ml scintillation fluid (Flow I, Molecular Diagnosis) and measuring the radioactivity of the mixture in a scintillation counter. The relative COMT enzyme activity is presented as DPM per mg total protein. To establish a baseline control for nonspecific reactions that do not depend on COMT, 5 μl of the specific COMT inhibitor, tolcapone (10 mg/ml), was added to a tube containing 100 μg of the sample. The high concentration of potent inhibitor will block the specific reaction catalyzed by COMT, and the radioactivity from this reaction served as a baseline. Each sample was assayed three times and the mean taken as the measure of COMT activity. COMT activity during the two placebo time points was highly correlated (ρ=0.90, p<0.0001, Spearman rank order correlation).
RESULTS
Demographic Information
Of the 51 volunteers who qualified for the study, four subjects dropped out from the study, thus 47 subjects were available for the final analysis. One male dropped out owing to gastrointestinal difficulties thought unrelated to tolcapone, and one female and one male dropped out before starting the compound for undisclosed reasons. A second female tested weakly positive for pregnancy at the end of her first arm (placebo) and her trial was discontinued. All 47 subjects were included in the neuropsychological testing analysis. However, only 34 were included for fMRI analysis and 39 for COMT enzyme assays. Subjects were excluded because they did not complete the entire fMRI study, or because of unavailability of the MRI scanner or their blood. All subjects confirmed urine discoloration throughout the trial. The most common side effects reported with tolcapone were dizziness (10%), nausea (10%), diarrhea (10%), loss of appetite (5%), stiffness (5%), sleep difficulties (5%), muscle spasms (2%), and irritability (2%). Similar reports occurred with placebo: that is nausea (10%), sleep difficulties (7%), loss of appetite (7%), diarrhea (7%), dizziness (7%), vomiting (2%), and stiffness (2%). In all cases, symptoms were mild and short lived with the exception of one subject in whom GI symptoms were moderate. In two subjects, liver enzyme levels doubled during the active phase of the protocol. LFTs rapidly normalized after discontinuing the compound.
The demographic characteristics of the 47 individuals who completed the study are presented in Table 1. Fifteen subjects had val/val genotypes, 21 were val/met, and 11 were met/met. Nonparametric analysis for gender revealed nonsignificant differences between genotypes (χ2=2.52, p=0.11, val/val vs val/met; χ2=2.69, p=0.10 val/met vs met/met; χ2=0.04, p=0.85 met/met vs val/val). ANOVA comparisons for age, education, or IQ revealed no significant differences between genotypes groups (Table 1).
COMT Enzyme Assay in Blood
Determination of whole-blood COMT activity on placebo (‘baseline’) showed that val/val individuals had higher enzyme activity when compared to val/met and met/met individuals (56 656±10 292 cpm/mg protein, val/val; 46 039± 9078 cpm/mg protein, val/met; 39 413±5126 cpm mg protein, met/met) (see Figure 1). These data are consistent with earlier reports of the effect of COMT genotype on red blood cell COMT activity (Syvanen et al, 1997). No gender differences in COMT activity were observed in the whole cohort or in the cohort stratified by genotype during the placebo phase. Analysis of variance of these data showed a highly significant effect of genotype on COMT activity (F(36)=9.29; p=0.0005). Post hoc analysis indicated that val/val individuals have greater activity than val/met subjects (p<0.001) and met/met individuals (p=0.001). Likely due to small sample size, there were no significant differences between the val/met and the met/met groups.
Repeated measures ANOVA of COMT activity determined 4 h after treatment (placebo vs tolcapone) revealed a highly significant drug effect (F(36)=84.9; p<0.0001) and a significant genotype × drug interaction (F(36)=11.80; p=0.0001) (Figure 1). Repeated measure ANOVA of COMT activity before the morning dose of medication revealed a main effect of genotype (P(36)=6.42, p=0.004) and of drug (F(36)=34.45, p=0.0001), but no drug × genotype interaction (F(36)=2.25, p=0.12). On average, however, tolcapone administration at this dose led to only a 25% reduction in peripheral COMT activity, a difference comparable to that found with COMT val/met genotype (33).
Neuropsychological Testing
Three of the tests of executive cognition showed positive effects of tolcapone. On the trail making test, a significant effect of tolcapone (F=4.64, df=1, p<0.05) was observed, ie individuals while on tolcapone performed the trail making test more rapidly than while on placebo (Figure 2a). No order (F=0.03, df=1, p>0.05) nor genotype (F=0.10, df=2, p>0.05) effects were present, nor was a genotype × drug interaction (F=0.18, df=2, p>0.05) found for this test. For the N-Back test, a drug × test interaction (F=2.8, df=3.30, p<0.05) was present on the RT measure, such that RT for 2- and 3-Back conditions improved with tolcapone, whereas RT for the less demanding 0- and 1-Back conditions showed little change. No genotype (F=0.24, df=2,10, p>0.05) or order (F=0.53, df=1.10, p>0.05) effects were present, nor was a genotype × drug interaction (F=0.02, df=2.10, p>0.05) found (Figure 2b). For intradimensional set shifting, a significant drug × genotype interaction (F=4.9, df=2, p<0.05) was present, such that individuals with val/val genotypes markedly improved while receiving tolcapone, whereas individuals with met/met genotypes worsened (Figure 2d). Tolcapone also showed a trend for the predicted effect on verbal episodic memory in the form of a drug × genotype interaction (F=2.34, df=2, p=0.1), such that individuals with val/val genotypes markedly improved while receiving tolcapone, whereas individuals with met/met genotypes worsened. Neither order effects were present (F=1.6, df=1, p>0.05) (Figure 2c). For none of these three tasks was either a main effect of drug status (F=1.45, df=1, p>0.05) or a main effect of genotype (F=2.08, df=2, p>0.05) found, likely because of these interaction effects. No order effects or other interactions were found.
As a further exploration of the specificity of these findings to tolcapone treatment per se, we also constructed a series of multiple regressions in order to assess whether variance in tolcapone-induced COMT inhibition independently contributed to variance in cognition effects. On–off difference scores for each cognitive measure served as the dependent variables. Genotype, percent COMT inhibition (derived from active drug vs placebo activity measures), and genotype × percent COMT inhibition served as the independent variables in each regression. Percent inhibition did not enter significantly (ie p<0.15) in any of the regressions. Thus, individual variations in COMT inhibition in peripheral blood were not a significant predictor of cognitive response to tolcapone, independent of the fact of drug administration. However, consistent with drug status × genotype interactions in the ANOVA analyses (see above), COMT genotype entered significantly as a predictor of verbal memory recall and ID shifting.
There were no changes in any of the POMS scales associated with tolcapone treatment. Specifically, scales for tension, vigor, anger, and fatigue did not differ significantly between the arms of the study (by matched pair t-test, all p>0.10).
Imaging Results
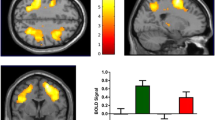
The spatial distribution of the N-Back-related activation responses analyzed as a main effect of task included PFC (BA 9-10/44-46), pericingulate cortex, anterior cingulate (BA 24, 32), and parietal cortex (BA 7, 39-40) bilaterally at all levels of task difficulty on both drug conditions. Analysis of variance revealed a significant main effect of drug on the efficiency of information processing during the task, with greater activation in brain regions subserving WM, particularly in the DLPFC (BA 9/46) bilaterally on placebo compared with tolcapone (p<0.02, SVC in right DLPFC; p<0.03, SVC in left DLPFC) (Figure 3a and b). This was observed in the absence of a significant difference in accuracy or RT across the two drug conditions (data not shown). Analysis in the reverse direction did not reveal hyperactivation in the PFC on Tolcapone relative to the placebo condition. These data are consistent with prior results based on COMT genotype in normal subjects and with our hypotheses based on these prior probabilities.
(a) Group activation map showing a significant main effect of drug (placebo>tolcapone) in the DLPFC (BA 9/46) bilaterally (p<0.02, SVC) consistent with a positive effect of tolcapone on functional efficiency. (b) Signal intensity plots from clusters of voxels in the left and right DLPFC that showed a main effect of drug (placebo>tolcapone, p<0.02, SVC). x-axis=drug, y-axis=normalized BOLD signal intensity.
COMMENT
We have found that tolcapone, a CNS penetrant COMT inhibitor (Ceravolo et al, 2002), enhances memory and executive cognition and the physiologic efficiency of prefrontal cortical information processing in normal human subjects. To our knowledge, this is the first evidence of a pharmacological enhancement of these functions in normal subjects without psychostimulant properties. Our data are consistent with the effects of a functional polymorphism in the COMT gene on similar cognitive and physiologic measures in normal subjects, and with some of the effects of amphetamine, an indirect monaminergic agonist with psychostimulant effects. Whereas biogenic aminergic stimulants tend to enhance DA, norepinephrine, and serotonin in widespread regions of brain, tolcapone relatively selectively enhances cortical extracellular DA levels (Tunbridge et al, 2004). These regionally restricted effects of COMT inhibition, which are consistent with the apparent lack of psychostimulant properties of tolcapone, suggest low potential for abuse and tolerance.
Our cognitive effects are also in line with previous clinical data on elderly patients with Parkinson's disease in which tolcapone administered over a 6-month period in combination with l-dopa was effective in improving working memory and attentional impairments, and these effects persisted even after tapering the dose of l-dopa (Gasparini et al, 1997). The maximum clinical effect of tolcapone in patients with Parkinson's disease was reported to occur during the first day of treatment and continued unchanged during the first week of treatment. Experimental studies in animals showed that tolcapone has a 60% brain bioavailability reaching a brain concentration to 1% of plasma concentrations (Forsberg et al, 2003). Tolcapone inhibits brain COMT with an ID50 of about 27 mg/kg reaching a 70% COMT activity inhibition within 30 min (Da Prada et al, 1991; Zurcher et al, 1991). In human volunteers, a 70% maximum inhibition of red blood cells COMT activity is observed at 2 h with a 60% inhibition at 4 h after an acute dose of 200 mg of tolcapone (Jorga, 1998). Previous studies of COMT activity in human red blood cells (Syvanen et al, 1997) and in human brain (Chen et al, 2004) have also indicated that COMT val/met genotype affects COMT activity, but the difference between homozygote genotypes is less than that associated with higher doses of tolcapone administration. Our data in peripheral blood on and off tolcapone are consistent with these various earlier reports. Interestingly, we found evidence that COMT genotype interacts with tolcapone-induced inhibition, such that val/val individuals may be more drug sensitive. This may reflect the greater capacity of the val allele to bind ligand because of its greater stability (Chen et al, 2004). However, these results involve small samples of met/met individuals and need further investigation.
A previous fMRI study showed that prefrontal DA receptor activation with amphetamine results in an improvement in the efficiency of PFC information processing during the same N back test in normal volunteers (Mattay et al, 2003). A similar effect of l-dopa therapy was found in patients with Parkinson's disease undergoing fMRI during the same task (Mattay et al, 2002). Efficiency is an operational measure of information processing based on the fMRI response during working memory challenge. In principle, the same behavioral output in the context of less cortical activation would imply more efficient processing of the information. In our study, tolcapone also improved DLPFC functional efficiency in normal volunteers. We did not, however, observe overall significant genotype by tolcapone effects on fMRI-based efficiency measures, likely because of the limited power of the small sample. Similarly, tolcapone improved frontal lobe function as revealed by several significant effects on tests of executive function, psychomotor speed and speed of processing, verbal memory, and measurements of intradimensional shifting. In these latter two tests, drug × genotype effects were observed, because of a particularly positive effect in val/val individuals. This drug by genotype effect is consistent with relatively lower prefrontal cortical DA signaling in these subjects and presumed benefit of enhanced prefrontal DA in this context (Mattay et al, 2003; Weinberger et al, 2001).
We observed main effects of tolcapone in tests that demand speed of response in the context of working memory, namely trail making (in which the sequencing of letters and numbers proceeds in an alternating fashion) and N Back at high loads in which updating of the working memory buffer is near continuous. For two other tasks, the response to tolcapone was modulated by COMT genotype. Intradimensional shifting requires rule generalization in the face of a salient distractor; we have found an effect of COMT genotype in a large sample of schizophrenic probands that are consistent with these results (Diaz-Asper et al, 2005). The COMT effect on episodic memory may reflect its effect on prefrontal cortical function, a region engaged during encoding of verbal information and/or engagement of the medial-temporal lobe thought to be involved in the consolidation of the memoranda. Regarding the latter possibility, in humans COMT is known to be abundantly expressed in medial temporal lobe regions, especially the hippocampus (Matsumoto et al, 2003). Furthermore, COMT genotype effects on a variant of an episodic memory task have been described in an aging adult population (de Frias et al, 2004). The presence of genotype effects on some tests of executive cognition but not others may indicate a more selective effect of tolcapone on circumscribed prefrontal processing circuits strictly related to those tasks. We cannot rule out, however, that the restricted genotype effects may be due to a lack of statistical power in our sample.
The magnitude of the effects of tolcapone on the various cognitive tests is small, consistent with earlier reports of the effects of COMT genotype in normal subjects on the same tests, which generally have involved even larger samples. There are undoubtedly many reasons for this, including the CNS potency of the drug and our relatively low dosing. The most important factor, however, is that these subjects are normal individuals without obvious deficits on the tasks used. Thus, there is a ‘ceiling effect’ on performance built into this study, making the positive effects much more remarkable. It is interesting to note that the effect sizes for those tests that demonstrated improvements in the tolcapone condition ranged from 0.23 to 0.51 with a mean of 0.34. Interestingly, we did not observe an effect of COMT on Wisconsin Card Sort (WCST) performance, consistent with evidence that COMT genotype appears not to reliably predict variation in WCST performance as it does in other measures, such as the N back and IDS stage of the ID/ED test (Diaz-Asper et al, 2005; Egan et al, 2001; Goldberg et al, 2003). The lack of an effect of tolcapone on WCST measures also may be confounded by a strong order effect with this task, such that taking it once dramatically alters the cognitive demands of subsequent trials.
It is important to acknowledge that we performed a number of tests in this study, based on earlier reports of COMT genotype effects on well-validated measures of executive cognition and episodic memory, and none of our cognitive results would survive statistical correction for multiple comparisons. We could have restricted our measures only to tests, for example N back and Trails, that have shown consistent earlier COMT genotype effects, but this did not seem appropriate to this ‘proof of concept’ pharmacological intervention. We do not believe that an agnostic correction for multiple testing is an appropriate procedure in this context because the tests were based on very strong prior probability of effects and are strikingly similar to what has been reported as the cognitive correlates of COMT genotype. In our normal population, it is reasonable to expect that in some of the tests measuring cognitive functions unrelated to COMT, tolcapone would not induce statistically significant changes. The lack of effect of tolcapone on COMT-unrelated functional measures provided further evidence on the specificity of the cognitive tests in which this drug had a statistically significant effect. By the same logic, differences in our results from earlier studies with amphetamine are not surprising, given the inevitability of cohort effects due to relatively small samples, and to the global effect of amphetamine on other biogenic amines and in brain regions other than cortex.
In contrast to the effects on cognition, the effect of tolcapone on an fMRI measure of prefrontal cortical efficiency during executive cognitive processing was clear and robust, and consistent with earlier main effects of amphetamine. These results are in line with evidence in experimental animals of a role of COMT in cortical DA signaling. Polymorphisms in the COMT gene contribute to the variability in the cortical responses to classic indirect dopaminergic agonists (Mattay et al, 2003). In fact, the effects of amphetamine on prefrontal efficiency measured with fMRI (Mattay et al, 2003) were more noticeable in val/val individuals, a group with putatively higher COMT activity in the frontal cortex and, hence, relatively lower DLFPC synaptic DA concentrations. In line with these findings, we expected that normal val/val individuals would have a differentially greater improvement in DLPFC physiological efficiency, working memory, and other cognitive tasks related to frontal cortex executive function after administration of tolcapone. To some degree, our neuropsychological data are consistent with this prediction. A significant drug × genotype effect was observed in measurements of intradimensional shifting and a trend towards significance of this interaction was found in verbal episodic memory, supporting the notion that trait differences in synaptic DA in the DLPFC may be responsible for this differential response between groups. We did not find, however, a significant interaction of genotype on fMRI measures, as previously reported with amphetamine. For a variety of reasons related to the differential pharmacology of these drugs, noted above, direct comparisons of their effects are limited.
The present study provides, to our knowledge, the first evidence of enhancement of executive cognition and frontal lobe function by a nonstimulant drug. The ability of tolcapone to improve executive cognitive function putatively by selectively increasing DA turnover in the prefrontal and possibly temporal cortices provides a unique example of ‘targeted’ pharmacology for the treatment of cognitive disorders. The possibility that there may be interactions with COMT genotype that modify these effects also illustrates that this novel pharmacological strategy may also be genotype targeted. Importantly, unlike psychostimulants, the improvement of cognitive function and frontal lobe physiologic efficiency in normal volunteers is not accompanied by changes in motor function or mood status, implicating a lack of effect of the drug on the mesolimbic and mesostriatal dopaminergic systems. This regionally selective effect is consistent with studies in animals (Gogos et al, 1998; Mazei et al, 2002; Tunbridge et al, 2004).
References
Ceravolo R, Piccini P, Bailey DL, Jorga KM, Bryson H, Brooks DJ (2002). 18F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson's disease. Synapse 43: 201–207.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807–821.
Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002). Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain 125: 584–594.
Da Prada M, Zurcher G, Kettler R, Colzi A (1991). New Therapeutic Strategies in Parkinson's Disease: Inhibition of MAO-B by Ro 19-6327 and of COMT by Ro 40-7592. Plenum Press: New York.
de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG (2004). COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet 34: 533–539.
D’Esposito M, Postle BR, Rypma B (2000). Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 133: 3–11.
Diaz-Asper C, Goldberg T, Kolachana B, Mazzanti C, Callicot J, Straub R et al (2005). Genetic variation in COMT effects on working memory function in Schizophrenic patients and healthy controls (submitted).
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917–6922.
Forsberg M, Lehtonen M, Heikkinen M, Savolainen J, Jarvinen T, Mannisto P (2003). Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat. J Pharmacol Exp Ther 304: 498–506.
Gasparini M, Fabrizio E, Bonifati V, Meco G (1997). Cognitive improvement during tolcapone treatment in Parkinson's disease. J Neural Transm 104: 887–894.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996.
Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR (1997). Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 54: 159–165.
Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS et al (2003). Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60: 889–896.
Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G et al (2000). A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 14: 353–360.
Guldberg HC, Marsden CA (1975). Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev 27: 135–206.
Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J et al (1999). Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. NeuroImage 9: 108–116.
Hardy GH (1908). Mendelian proportions in a mixed population. Science 28: 49–50.
Ho BC, Wassink TH, O’Leary DS, Sheffield VC, Andreasen NC (2005). Catechol-O-methyl transferase Val(158)Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry 10: 287–298.
Jorga K, Fotteler B, Heizmann P, Gasser R (1999). Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyltransferase. Br J Clin Pharmacol 48: 513–520.
Jorga KM (1998). Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology 50: S31–S38.
Karoum F, Chrapusta SJ, Egan MF (1994). 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem 63: 972–979.
Li YH, Wirth T, Huotari M, Laitinen K, MacDonald E, Mannisto PT (1998). No change of brain extracellular catecholamine levels after acute catechol-O-methyltransferase inhibition: a microdialysis study in anaesthetized rats. Eur J Pharmacol 356: 127–137.
Liljequist R, Haapalinna A, Ahlander M, Li YH, Mannisto PT (1997). Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav Brain Res 82: 195–202.
Lloyd KG, Davidson L, Hornykiewicz O (1975). The neurochemistry of Parkinson's disease: effect of L-dopa therapy. J Pharmacol Exp Ther 195: 453–464.
Mannisto PT, Kaakkola S (1999). Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51: 593–628.
Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM et al (2003). Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 28: 1521–1530.
Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R et al (2000). Effects of dextroamphetamine on cognitive performance and cortical activation. NeuroImage 12: 268–275.
Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF et al (2003). Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 100: 6186–6191.
Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN et al (2002). Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol 51: 156–164.
Mazei MS, Pluto CP, Kirkbride B, Pehek EA (2002). Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936: 58–67.
McNair D, Lorr M (1981). ITS Manual for the Profile of Mood States. Multi-health System, Inc.: San Diego, CA.
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389–395.
Pozzi L, Invernizzi R, Cervo L, Vallebuona F, Samanin R (1994). Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem 63: 195–200.
Seamans JK, Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18: 2697–2708.
Syvanen AC, Tilgmann C, Rinne J, Ulmanen I (1997). Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics 7: 65–71.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004). Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24: 5331–5335.
Weinberg W (1908). ber den Nachweis der Vererbung beim Menschen. Jahresh Ver Vaterl Naturk Württemb 64: 368–382.
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK et al (2001). Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50: 825–844.
Zurcher G, Colzi A, Da Prada M (1990a). Ro 40-7592: inhibition of COMT in rat brain and extracerebral tissues. J Neural Transm Suppl 32: 375–380.
Zurcher G, Dingemanse J, Da Prada M (1991). Ro-40-7592, A Potent Inhibitor of Extracerebral and Brain Catechol-O-methyltransferase: Preclinical and Clinical Findings. John Libbey SRL: Rome.
Zurcher G, Keller HH, Kettler R, Borgulya J, Bonetti EP, Eigenmann R et al (1990b). Ro 40-7592, a novel, very potent, and orally active inhibitor of catechol-O-methyltransferase: a pharmacological study in rats. Adv Neurol 53: 497–503.
Acknowledgements
We thank the following persons for their excellent assistance: Ben Kosiorowski, Julie Kohn, Francis Glenn, Mary Weirich, Denise Niner, Saumitra Das, Cayenne Nikoosh Carlo, Gerald Overman, and the 4-E Unit nursing staff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
No author has conflict of interests related to the present research.
Rights and permissions
About this article
Cite this article
Apud, J., Mattay, V., Chen, J. et al. Tolcapone Improves Cognition and Cortical Information Processing in Normal Human Subjects. Neuropsychopharmacol 32, 1011–1020 (2007). https://doi.org/10.1038/sj.npp.1301227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301227
Keywords
This article is cited by
-
Genetic profile for dopamine signaling predicts brain functional reactivity to repetitive transcranial magnetic stimulation
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Evidence for anti-inflammatory effects and modulation of neurotransmitter metabolism by Salvia officinalis L.
BMC Complementary Medicine and Therapies (2022)
-
Catechol-O-Methyltransferase Effects on Smoking: a Review and Proof of Concept of Sex-Sensitive Effects
Current Behavioral Neuroscience Reports (2022)
-
Novel, non-nitrocatechol catechol-O-methyltransferase inhibitors modulate dopamine neurotransmission in the frontal cortex and improve cognitive flexibility
Psychopharmacology (2020)
-
Estrogen Signaling in Alzheimer’s Disease: Molecular Insights and Therapeutic Targets for Alzheimer’s Dementia
Molecular Neurobiology (2020)