Abstract
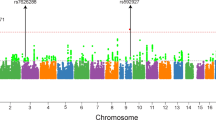
Schizophrenia is a complex neuropsychiatric disorder to which an as-yet-unknown number of genes contribute, interacting with each other and the environment. Linkage analyses have implicated several chromosomal regions as harboring schizophrenia susceptibility loci although rarely at levels commensurate with proposed thresholds for genome-wide significance. We systematically recruited Arab Israeli families multiply affected with schizophrenia from the catchment area of a Regional Mental Health Center. Clinical diagnoses were established by semistructured interviews and all other available sources of information under narrow, core and broad categories. Using 350 microsatellite markers, spaced at an average of 10.3 cM, we performed an autosomal scan in 155 subjects from 21 families. Linkage analysis employed affects only, multipoint, nonparametric (model-free) and also parametric (dominant and recessive) approaches. We detected significant evidence for a schizophrenia susceptibility gene at chromosome 6q23 with a nonparametric LOD score (NPL) of 4.60 (P=0.000004) under the broad diagnostic category and a parametric LOD score of 3.33 (dominant model). Under the core diagnostic category the NPL was 4.29 (P=0.00001) and the LOD score 4.16 (dominant model). We also detected suggestive evidence for linkage at chromosome 10q24 under the broad diagnostic category (NPL 3.24, P=0.0008; heterogeneity LOD score, dominant model 2.65, α=0.82). Additionally, NPL scores >2.0 were observed at chromosome 2q37, 4p15–16, 7p22, 9q21–22 and 14q11.1–11.2. The linkage we detected at chromosome 6q23 fulfills the criteria for genome-wide significance and is located approximately midway between loci suggested by a previous significant report at chromosome 6q25 and findings located more centromerically at 6q21–22.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baron M . Genetics of schizophrenia and the new millennium: progress and pitfalls. Am J Hum Genet 2001; 68: 299–312.
Kohn Y, Lerer B . Genetics of schizophrenia: a review of linkage findings. Isr J Psychiatry Rel Sci 2002; 39: 240–321.
Badner JA, Gershon ES . Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7: 405–411.
Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 2002; 71: 337–348.
Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002; 71: 877–892.
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 2002; 99: 13675–13680.
Jaber L, Bailey-Wilson JE, Haj-Yehia M, Hernandez J, Shohat M . Consanguineous matings in an Israeli-Arab community. Arch Pediatr Adolesc Med 1994; 148: 412–415.
Jaber L, Halpern GJ, Shohat T . Trends in the frequencies of consanguineous marriages in the Israeli Arab community. Clin Genet 2000; 58: 106–110.
Spitzer R, Endicott J (eds.) The Schedule for Affective Disorders and Schizophrenia, Lifetime Version, 3rd edn. New York State Psychiatric Institute: New York, 1977.
Andreasen NC, Endicott J, Spitzer RL, Winokur G . The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34: 1229–1235.
Spitzer RL, Endicott J, Robins E . Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35: 773–782.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association: Washington, DC, 1994.
Baron M, Endicott J, Lerer B, Loth JE, Alexander JR, Simon R et al. A pedigree series for mapping disease genes in bipolar affective disorder: sampling, assessment and analytic considerations. Psychiatr Genet 1994; 4: 43–55.
Ewen KR, Bahlo M, Treloar SA, Levinson DF, Mowry B, Barlow JW . Identification and analysis of error types in high-throughput genotyping. Am J Hum Genet 2000; 67: 727–736.
Gudbjartsson DF, Jonasson K, Frigge ML, Kong A . Allegro, a new computer program for multipoint linkage analysis. Nat Genet 2000; 25: 12–13.
Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES . Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 1996; 58: 1347–1363.
Whittemore AS, Halpern J . Problems in the definition, interpretation, and evaluation of genetic heterogeneity. Am J Hum Genet 2001; 68: 457–465.
Hodge SE, Vieland VJ, Greenberg DA . HLODs remain powerful tools for detection of Linkage in the presence of genetic heterogeneity. Am J Hum Genet 2002; 70: 556–557.
Kong A, Cox NJ . Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 1997; 61: 1179–1188.
Lander E, Kruglyak L . Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11: 241–247.
Cardon LR, Bell JI . Association study designs for complex traits. Nat Rev Genet 2001; 2: 91–99.
Cao Q, Martinez M, Zhang J, Sanders AR, Badner JA, Crzvchik A et al. Suggestive evidence for a schizophrenia susceptibility locus on chromosome 6q and a confirmation in an independent series of pedigrees. Genomics 1997; 43: 1–8.
Martinez M, Goldin LR, Cao Q, Zhang J, Sanders AR,, Nancarrow DJ et al. Follow-up study on a susceptibility locus for schizophrenia on chromosome 6q. Am J Med Genet 1999; 88: 337–343.
Levinson DF, Holmans P, Straub RE, Owen MJ, Wildenauer DB, Gejman PV et al. Multicenter linkage study of schizophrenia candidate regions on chromosomes 5q, 6q, 10p, and 13q: schizophrenia linkage collaborative group III. Am J Hum Genet 2000; 67: 652–663.
Lindholm E, Ekholm B, Shaw S, Jalonen P, Johansson G, Pettersson U et al. A schizophrenia-susceptibility locus at 6q25, in one of the world's largest reported pedigrees. Am J Hum Genet 2001; 69: 96–105.
Bailer U, Leisch F, Meszaros K, Lenzinger E, Willinger U, Strobl R et al. Genome scan for susceptibility loci for schizophrenia. Neuropsychobiology 2000; 42: 175–182.
Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD et al. NIMH Genetics Initiative Millenium Schizophrenia Consortium: linkage analysis of African-American pedigrees. Am J Med Genet 1998; 81: 282–289.
Roberts SB, MacLean CJ, Neale MC, Eaves LJ, Kendler KS . Replication of linkage studies of complex traits: an examination of variation in location estimates. Am J Hum Genet 1999; 65: 876–884.
Levinson DF, Mahtani MM, Nancarrow DJ, Brown DM, Kruglyak L, Kirby A et al. Genome scan of schizophrenia. Am J Psychiatry 1998; 155: 741–750.
Mowry BJ, Ewen KR, Nancarrow DJ, Lennon DP, Nertney DA, Jones HL et al. Second stage of a genome scan of schizophrenia: study of five positive regions in an expanded sample. Am J Med Genet 2000; 96: 864–869.
Ewald E, Flint TJ, Jorgensen TH, Wang AG, Jensen P, Vang M et al. Search for shared segment on chromosome 10q26 in patients with bipolar affective disorder or schizophrenia from the Faroe Islands. Am J Med Genet 2002; 114: 196–204.
Cichon S, Schmidt-Wolf G, Schumacher J, Muller DJ, Hurte M, Schulze TG et al. A susceptibility locus for bipolar affective disorder in chromosomal region 10q25–q26. Mol Psychiatry 2001; 6: 342–349.
Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnicki AD, Remick RA et al. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci USA 2001; 98: 585–590.
Paunio T, Ekelund J, Varilo T, Parker A, Hovatta I, Turunen JA et al. Genome-wide scan in a nationwide study sample of schizophrenia families in Finland reveals susceptibility loci on chromosomes 2q and 5q. Hum Mol Genet 2001; 10: 3037–3048.
Faraone SV, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B et al. Genome scan of European-American schizophrenia pedigrees: results of the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet 1998; 81: 290–295.
DeLisi LE, Shaw SH, Crow TJ, Shields G, Smith AB, Larach VW et al. A genome-wide scan for linkage to chromosomal regions in 382 sibling pairs with schizophrenia or schizoaffective disorder. Am J Psychiatry 2002; 159: 803–812.
Akel M, Masarwi UJ . Taibe Bani Saab: Past Present and Future. The Center for Arab Culture and Development, Alrama Publications: Israel, 1989, pp 51–78.
Israel Central Bureau of Statistics. Census of Population and Housing. Publication No. 10. Israel Central Bureau of Statistics: Jerusalem, 1961.
Israel Central Bureau of Statistics. Census of Population and Housing. Publication No. 5. Israel Central Bureau of Statistics: Jerusalem, 1972.
Israel Central Bureau of Statistics. Census of Population and Housing. Publication No. 7(1). Israel Central Bureau of Statistics: Jerusalem, 1995.
Jaber L, Merlob P, Bu X, Rotter JI, Shohat M . Marked parental consanguinity as a cause for increased major malformations in an Israeli Arab community. Am J Med Genet 1992; 44: 1–6.
Fallin D, Cohen A, Essioux L, Chumakov I, Blumenfeld M, Cohen D et al. Genetic analysis of case/control data using estimated haplotype frequencies: application to APO-E locus variation and Alzheimer's disease. Genome Res 2001; 11: 143–151.
Tsuang MT . ‘Schizoaffective disorder’: dead or alive? Arch Gen Psychiatry 1979; 36: 633–634.
Gershon ES, DeLisi LE, Hamovit J, Nurnberger Jr JI, Maxwell ME, Schreiber J et al. A controlled family study of chronic psychoses. Schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45: 328–336.
Berrettini WH . Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry 2000; 48: 531–538.
Pulver AE, Mulle J, Nestadt G, Swartz KL, Blouin JL, Dombroski B et al. Genetic heterogeneity in schizophrenia: stratification of genome scan data using co-segregating related phenotypes. Mol Psychiatry 2000; 5: 650–653.
Acknowledgements
This work was supported in part by internal funds from Hadassah Medical Organisation and by the German Israeli Foundation for Scientific Research (to BL). FM, NARSAD 2001 Essel Investigator, was partially supported by an Investigator Initiated Grant from the National Alliance for Research on Schizophrenia and Depression (NARSAD), USA. The Biological Psychiatry Laboratory, Hadassah—Hebrew University Medical Center, is supported by the Harry Stern Family Foundation. The authors are indebted to the late Mrs Blanche Stern Prince and to Dr Morton Prince for their encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerer, B., Segman, R., Hamdan, A. et al. Genome scan of Arab Israeli families maps a schizophrenia susceptibility gene to chromosome 6q23 and supports a locus at chromosome 10q24. Mol Psychiatry 8, 488–498 (2003). https://doi.org/10.1038/sj.mp.4001322
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001322
Keywords
This article is cited by
-
Molecular Variants in Human Trace Amine-Associated Receptors and Their Implications in Mental and Metabolic Disorders
Cellular and Molecular Neurobiology (2020)
-
Effect of chronic unpredictable stress on mice with developmental under-expression of the Ahi1 gene: behavioral manifestations and neurobiological correlates
Translational Psychiatry (2018)
-
Alterations in the expression of a neurodevelopmental gene exert long-lasting effects on cognitive-emotional phenotypes and functional brain networks: translational evidence from the stress-resilient Ahi1 knockout mouse
Molecular Psychiatry (2017)
-
Neural mechanisms underlying stress resilience in Ahi1 knockout mice: relevance to neuropsychiatric disorders
Molecular Psychiatry (2014)
-
Analysis of 10 independent samples provides evidence for association between schizophrenia and a SNP flanking fibroblast growth factor receptor 2
Molecular Psychiatry (2009)