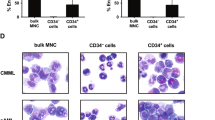
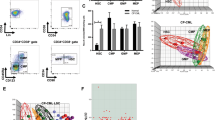
Abstract
We investigated the constitutive activation of the MEK/ERK pathway in acute myelogenous leukemia (AML) via a flow cytometric technique to quantitate expression of phosphorylated ERK (p-ERK). A total of 42 AML samples (16 newly diagnosed, 26 relapsed/refractory) were analyzed. Normal bone marrow CD34+ cells (n=10) had little or no expression of p-ERK, while G-CSF-mobilized CD34+ cells exhibited enhanced p-ERK levels. Markedly elevated p-ERK levels were found in 83.3% of the AML samples, with no differences observed between the newly diagnosed and relapsed/refractory samples. Treatment with a MEK inhibitor resulted in significantly decreased p-ERK levels in both the newly diagnosed and relapsed/refractory samples, which was associated with growth arrest, but not apoptosis induction. In summary, we defined conditions for the analysis of MAPK signaling in primary AML samples. Normal CD34+ cells expressed very low levels of p-ERK, and increased p-ERK levels were found in normal G-CSF-stimulated circulating CD34+ cells. Constitutively high p-ERK levels observed in the majority of AML samples suggest deregulation of this pathway that appears to be independent of disease status. The ability of ERK inhibition to promote growth arrest rather than apoptosis suggests that clinical trials of MEK/ERK inhibitors may be more effective when combined with chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ketley NJ, Allen PD, Kelsey SM, Newland AC . Mechanisms of resistance to apoptosis in human AML blasts: the role of differentiation-induced perturbations of cell-cycle checkpoints. Leukemia 2000; 14: 620–628.
Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003; 101: 2125–2131.
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003; 17: 1263–1293.
Platanias LC . Map kinase signaling pathways and hematologic malignancies. Blood 2003; 101: 4667–4679.
Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest 2001; 108: 851–859.
Dancey J, Sausville EA . Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2003; 2: 296–313.
Sebolt-Leopold JS . Development of anticancer drugs targeting the MAP kinase pathway. Oncogene 2000; 19: 6594–6599.
Allen LF, Sebolt-Leopold J, Meyer MB . CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK). Semin Oncol 2003; 30: 105–116.
Chow S, Patel H, Hedley DW . Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry 2001; 46: 72–78.
Krutzik PO, Nolan GP . Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry 2003; 55A: 61–70.
Perez OD, Nolan GP . Simultaneous measurement of multiple active kinase states using polychromatic flow cytometry. Nat Biotechnol 2002; 20: 155–162.
Tazzari PL, Cappellini A, Bortul R, Ricci F, Billi AM, Tabellini G et al. Flow cytometric detection of total and serine 473 phosphorylated Akt. J Cell Biochem 2002; 86: 704–715.
Fleisher TA, Dorman SE, Anderson JA, Vail M, Brown MR, Holland SM . Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin Immunol 1999; 90: 425–430.
Zell T, Khoruts A, Ingulli E, Bonnevier JL, Mueller DL, Jenkins MK . Single-cell analysis of signal transduction in CD4T cells stimulated by antigen in vivo. Proc Natl Acad Sci USA 2001; 98: 10805–10810.
Birkenkamp KU, Esselink MT, Kruijer W, Vellenga E . An inhibitor of PI3-K differentially affects proliferation and IL-6 protein secretion in normal and leukemic myeloid cells depending on the stage of differentiation. Exp Hematol 2000; 28: 1239–1249.
Fichelson S, Freyssinier JM, Picard F, Fontenay-Roupie M, Guesnu M, Cherai M et al. Megakaryocyte growth and development factor-induced proliferation and differentiation are regulated by the mitogen-activated protein kinase pathway in primitive cord blood hematopoietic progenitors. Blood 1999; 94: 1601–1613.
Lemoli RM, Tafuri A, Fortuna A, Petrucci MT, Ricciardi MR, Catani L et al. Cycling status of CD34+ cells mobilized into peripheral blood of healthy donors by recombinant human granulocyte colony-stimulating factor. Blood 1997; 89: 1189–1196.
Verfaillie CM, Almeida-Porada G, Wissink S, Zanjani ED . Kinetics of engraftment of CD34(−) and CD34(+) cells from mobilized blood differs from that of CD34(−) and CD34(+) cells from bone marrow. Exp Hematol 2000; 28: 1071–1079.
Steidl U, Kronenwett R, Rohr UP, Fenk R, Kliszewski S, Maercker C et al. Gene expression profiling identifies significant differences between the molecular phenotypes of bone marrow-derived and circulating human CD34+ hematopoietic stem cells. Blood 2002; 99: 2037–2044.
Lemoli RM, Bertolini F, Petrucci MT, Gregorj C, Ricciardi MR, Fogli M et al. Functional and kinetic characterization of granulocyte colony-stimulating factor-primed CD34− human stem cells. Br J Haematol 2003; 123: 720–729.
Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H . Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia 1997; 11: 479–484.
Lunghi P, Tabilio A, Dall'Aglio PP, Ridolo E, Carlo-Stella C, Pelicci PG et al. Downmodulation of ERK activity inhibits the proliferation and induces the apoptosis of primary acute myelogenous leukemia blasts. Leukemia 2003; 17: 1783–1793.
Cheng M, Sexl V, Sherr CJ, Roussel MF . Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc Natl Acad Sci USA 1998; 95: 1091–1096.
Hoshino R, Tanimura S, Watanabe K, Kataoka T, Kohno M . Blockade of the extracellular signal-regulated kinase pathway induces marked G1 cell cycle arrest and apoptosis in tumor cells in which the pathway is constitutively activated: upregulation of p27(Kip1). J Biol Chem 2001; 276: 2686–2692.
Balmanno K, Cook SJ . Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 1999; 18: 3085–3097.
Lobenhofer EK, Huper G, Iglehart JD, Marks JR . Inhibition of mitogen-activated protein kinase and phosphatidylinositol 3-kinase activity in MCF-7 cells prevents estrogen-induced mitogenesis. Cell Growth Differ 2000; 11: 99–110.
Kamakura S, Moriguchi T, Nishida E . Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem 1999; 274: 26563–26571.
Mody N, Leitch J, Armstrong C, Dixon J, Cohen P . Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett 2001; 502: 21–24.
Squires MS, Nixon PM, Cook SJ . Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem J 2002; 366: 673–680.
Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M . Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 2003; 102: 972–980.
Zhao S, Konopleva M, Cabreira-Hansen M, Xie Z, Hu W, Milella M et al. Inhibition of phosphatidylinositol 3-kinase (PI3K) dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia 2004; 18: 267–275.
Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG . Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res 2000; 6: 4885–4892.
MacKeigan JP, Taxman DJ, Hunter D, Earp III HS, Graves LM, Ting JP . Inactivation of the antiapoptotic phosphatidylinositol 3-kinase–Akt pathway by the combined treatment of taxol and mitogen-activated protein kinase kinase inhibition. Clin Cancer Res 2002; 8: 2091–2099.
Wilkinson E . Surprise phase III failure for ZD1839. Lancet Oncol 2002; 3: 583.
Blalock WL, Navolanic PM, Steelman LS, Shelton JG, Moye PW, Lee JT et al. Requirement for the PI3K/Akt pathway in MEK1-mediated growth and prevention of apoptosis: identification of an Achilles heel in leukemia. Leukemia 2003; 17: 1058–1067.
Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PW et al. Effects of the RAF/MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene 2003; 22: 2478–2492.
Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A et al. Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood 2002; 99: 3461–3464.
Milella M, Precupanu C, Gregorj C, Ricciardi M, Petrucci M, Kornblau SM et al. Beyond single pathway inhibition: MEK inhibitors as a platform for the development of pharmacological combinations with synergistic anti-leukemic effects. Curr Pharm Des, in press.
Acknowledgements
This work was supported in part by grants from the National Cancer Institute (PO1 CA55164 and CA16672) (to MA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
Supplementary information
Rights and permissions
About this article
Cite this article
Ricciardi, M., McQueen, T., Chism, D. et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia 19, 1543–1549 (2005). https://doi.org/10.1038/sj.leu.2403859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403859
Keywords
This article is cited by
-
Combination of RSK inhibitor LJH-685 and FLT3 inhibitor FF-10101 promoted apoptosis and proliferation inhibition of AML cell lines
Cellular Oncology (2022)
-
TNFAIP8 promotes AML chemoresistance by activating ERK signaling pathway through interaction with Rac1
Journal of Experimental & Clinical Cancer Research (2020)
-
Pan-RAF inhibition induces apoptosis in acute myeloid leukemia cells and synergizes with BCL2 inhibition
Leukemia (2020)
-
Anti-leukemic effects of simvastatin on NRASG12D mutant acute myeloid leukemia cells
Molecular Biology Reports (2019)
-
The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression
Leukemia (2017)