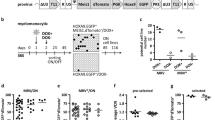
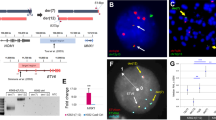
Abstract
Hox genes have been identified in chromosomal translocations involving the nucleoporin gene NUP98. Though the resulting chimeric proteins directly participate in the development of leukemia, the long latency and monoclonal nature of the disease support the requirement for secondary mutation(s), such as those leading to overexpression of Meis1. Models to identify such events and to study leukemic progression are rare and labor intensive. Herein, we took advantage of the strong transforming potential of NUP98–HOXD13 or NUP98–HOXA10 to establish preleukemic myeloid lines from bone marrow cells that faithfully replicate the first step of Hox-induced leukemogenesis. These lines contain early granulomonocytic progenitors with extensive in vitro self-renewal capacity, short-term myeloid repopulating activity and low propensity for spontaneous leukemic conversion. We exploit such lines to show that Meis1 efficiently induces their leukemic progression and demonstrate a high frequency of preleukemic cells in the cultures. Furthermore, we document that the leukemogenic potential of Meis1 is independent of its direct binding to DNA and likely reflects its ability to increase the repopulating capacity of the preleukemic cells by increasing their self-renewal/proliferative capacity. The availability of lines with repopulating potential and capacity for leukemic conversion should open new avenues for understanding progression of Hox-mediated acute myeloid leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Owens BM, Hawley RG . HOX and non-HOX homeobox genes in leukemic hematopoiesis. Stem Cells 2002; 20: 364–379.
Barbouti A, Hoglund M, Johansson B, Lassen C, Nilsson PG, Hagemeijer A et al. A novel gene, MSI2, encoding a putative RNA-binding protein is recurrently rearranged at disease progression of chronic myeloid leukemia and forms a fusion gene with HOXA9 as a result of the cryptic t(7;17)(p15;q23). Cancer Res 2003; 63: 1202–1206.
Lam DH, Aplan PD . NUP98 gene fusions in hematologic malignancies. Leukemia 2001; 15: 1689–1695.
Pineault N, Abramovich C, Ohta H, Humphries RK . Differential and common leukemogenic potentials of multiple NUP98–Hox fusion proteins alone or with Meis1. Mol Cell Biol 2004; 24: 1907–1917.
Sauvageau G, Thorsteinsdottir U, Hough MR, Hugo P, Lawrence HJ, Largman C et al. Overexpression of HOXB3 in hematopoietic cells causes defective lymphoid development and progressive myeloproliferation. Immunity 1997; 6: 13–22.
Kroon E, Thorsteinsdottir U, Mayotte N, Nakamura T, Sauvageau G . NUP98–HOXA9 expression in hemopoietic stem cells induces chronic and acute myeloid leukemias in mice. EMBO J 2001; 20: 350–361.
Buske C, Humphries RK . Homeobox genes in leukemogenesis. Int J Hematol 2000; 71: 301–308.
Pineault N, Buske C, Feuring-Buske M, Abramovich C, Rosten P, Hogge DE et al. Induction of acute myeloid leukemia in mice by the human leukemia-specific fusion gene NUP98–HOXD13 in concert with Meis1. Blood 2003; 101: 4529–4538.
Ayton PM, Cleary ML . Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 2001; 20: 5695–5707.
Thorsteinsdottir U, Kroon E, Jerome L, Blasi F, Sauvageau G . Defining roles for HOX and MEIS1 genes in induction of acute myeloid leukemia. Mol Cell Biol 2001; 21: 224–234.
Pineault N, Helgason CD, Lawrence HJ, Humphries RK . Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Exp Hematol 2002; 30: 49–57.
Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J 2004; 23: 450–459.
Fujino T, Yamazaki Y, Largaespada DA, Jenkins NA, Copeland NG, Hirokawa K et al. Inhibition of myeloid differentiation by Hoxa9, Hoxb8, and Meis homeobox genes. Exp Hematol 2001; 29: 856–863.
Calvo KR, Knoepfler PS, Sykes DB, Pasillas MP, Kamps MP . Meis1a suppresses differentiation by G-CSF and promotes proliferation by SCF: potential mechanisms of cooperativity with Hoxa9 in myeloid leukemia. Proc Natl Acad Sci USA 2001; 98: 13120–13125.
Hawley RG, Fong AZ, Lu M, Hawley TS . The HOX11 homeobox-containing gene of human leukemia immortalizes murine hematopoietic precursors. Oncogene 1994; 9: 1–12.
Calvo KR, Sykes DB, Pasillas MP, Kamps MP . Nup98-HoxA9 immortalizes myeloid progenitors, enforces expression of Hoxa9, Hoxa7 and Meis1, and alters cytokine-specific responses in a manner similar to that induced by retroviral co-expression of Hoxa9 and Meis1. Oncogene 2002; 21: 4247–4256.
Calvo KR, Sykes DB, Pasillas M, Kamps MP . Hoxa9 immortalizes a granulocyte-macrophage colony-stimulating factor-dependent promyelocyte capable of biphenotypic differentiation to neutrophils or macrophages, independent of enforced meis expression. Mol Cell Biol 2000; 20: 3274–3285.
Knoepfler PS, Sykes DB, Pasillas M, Kamps MP . HoxB8 requires its Pbx-interaction motif to block differentiation of primary myeloid progenitors and of most cell line models of myeloid differentiation. Oncogene 2001; 20: 5440–5448.
Shanmugam K, Green NC, Rambaldi I, Saragovi HU, Featherstone MS . PBX and MEIS as non-DNA-binding partners in trimeric complexes with HOX proteins. Mol Cell Biol 1999; 19: 7577–7588.
Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G . Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J 1998; 17: 3714–3725.
Delaney C, Bernstein ID . Establishment of a pluripotent preleukaemic stem cell line by expression of the AML1–ETO fusion protein in Notch1-immortalized HSCN1cl10 cells. Br J Haematol 2004; 125: 353–357.
Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM . Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol 1995; 15: 5434–5443.
Kawagoe H, Humphries RK, Blair A, Sutherland HJ, Hogge DE . Expression of HOX genes, HOX cofactors, and MLL in phenotypically and functionally defined subpopulations of leukemic and normal human hematopoietic cells. Leukemia 1999; 13: 687–698.
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF . Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 2004; 14: 43–47.
Shen WF, Montgomery JC, Rozenfeld S, Moskow JJ, Lawrence HJ, Buchberg AM et al. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol 1997; 17: 6448–6458.
Shen WF, Rozenfeld S, Kwong A, Kom ves LG, Lawrence HJ, Largman C . HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol Cell Biol 1999; 19: 3051–3061.
Kurant E, Eytan D, Salzberg A . Mutational analysis of the Drosophila homothorax gene. Genetics 2001; 157: 689–698.
Ryoo HD, Marty T, Casares F, Affolter M, Mann RS . Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex. Development 1999; 126: 5137–5148.
Acknowledgements
This work was supported in part by the National Cancer Institute of Canada, with funds from the Terry Fox Foundation, and Genome Canada/British Columbia. We thank Dr Bob Argiropoulos for helpful discussions, Patty Rosten for technical assistance, and Tara Palmater and Colleen MacKinnon for help with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pineault, N., Abramovich, C. & Humphries, R. Transplantable cell lines generated with NUP98–Hox fusion genes undergo leukemic progression by Meis1 independent of its binding to DNA. Leukemia 19, 636–643 (2005). https://doi.org/10.1038/sj.leu.2403696
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403696
Keywords
This article is cited by
-
Mutated NPM1 in combination with overexpression of Meis1 or Hoxa9 is not sufficient to induce acute myeloid leukemia
Experimental Hematology & Oncology (2015)
-
Regulation of MEIS1 by distal enhancer elements in acute leukemia
Leukemia (2014)
-
High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays
Nature Methods (2011)
-
HOX genes: not just myeloid oncogenes any more
Leukemia (2005)