Abstract
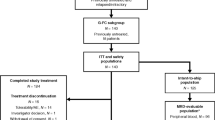
Patients with CLL responding to initial chemotherapy with fludarabine alone (F) or in combination with cyclophosphamide (FC) were randomized for treatment with alemtuzumab (30 mg i.v. TIW, 12 weeks) or observation. Of 21 evaluable patients, 11 were randomized to alemtuzumab before the study was stopped due to severe infections in seven of 11 patients. These infections (one life-threatening pulmonary aspergillosis IV; four CMV reactivations III requiring i.v. ganciclovir; one pulmonary tuberculosis III; one herpes zoster III) were successfully treated and not associated with cumulative dose of alemtuzumab. In the observation arm, one herpes zoster infection II and one sinusitis I were documented. At 6 months after randomization, two patients in the alemtuzumab arm converted to CR, while three patients in the observation arm progressed. After alemtuzumab treatment, five of six patients achieved a molecular remission in peripheral blood while all patients in the observation arm remained MRD-positive (P=0.048). At 21.4 months median follow-up, patients receiving alemtuzumab showed a significant longer progression-free survival (no progression vs mean 24.7 months; P=0.036). In conclusion, a consolidation therapy with alemtuzumab is able to achieve molecular remissions and longer survival in CLL, but a safe treatment regimen needs to be determined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1750–1757.
Leporrier M, Chevret S, Cazin B, Boudjerra N, Feugier P, Desablens B et al. Randomized comparison of fludarabine, CAP and CHOP, in 938 previously treated stage B and C chronic lymphocytic leukemia. Blood 2001; 98: 2319–2325.
O'Brien SM, Kantarjian HM, Cortes J, Beran M, Koller CA, Giles FJ et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol 2001; 19: 1414–1420.
Hallek M, Schmitt B, Wilhelm M, Busch R, Krober A, Fostitsch HP et al. Fludarabine plus cyclophospamide is an efficient treatment for advanced chronic lymphocytic leukemia: results of a phase II study of the German CLL Study Group. Br J Hematol 2001; 11: 1–8.
Dreger P, Montserrat E . Autologous and allogeneic stem cell transplantation for chronic lymphocytic leukemia. Leukemia 2002; 16: 985–992.
Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003; 101: 6–14.
Hale G, Dyer MJ, Clark MR, Phillips JM, Marcus R, Riechmann L et al. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet 1988; 2: 1394–1399.
Osterborg A, Dyer MJ, Bunjes D, Panglias GA, Bastion Y, Catovsky D et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH 1 H treatment in chronic lymphocytic leukemia. J Clin Oncol 1997; 15: 1567–1574.
Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A et al. CAMPATH-1 H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1 H Treatment in low-grade non-hodgkin's lymphoma. J Clin Oncol 1998; 16: 3257–3263.
Keating MJ, Rai K, Flinn I, Jain V, Binet JL, Hillmen P et al. Therapeutic role of alemtuzumab (Campath-1 H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H . Humanized CD52 monoclonal antibody Campath-1 H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol 1996; 93: 151–153.
Lundin J, Kimby E, Björkholm M, Broliden PA, Celsing F, Hjalmar V et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1 H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002; 100: 768–773.
Dyer MJ, Kelsey SM, Mackay HJ, Emmett E, Thornton P, Hale G et al. In vivo ‘purging’ of residual disease in CLL with CAMPATH 1-H. Br J Haematol 1997; 97: 669–672.
Heit W, Bunjes D, Wiesneth M, Schmeiser T, Arnold R, Hale G et al. Ex vivo T-cell depletion with the monoclonal antibody Campath-1 H plus human complement effectively prevents acute graft-versus-host disease in allogeneic bone marrow transplantation. Br J Haematol 1986; 64: 479–486.
Riechmann L, Clark M, Waldmann H, Winter G . Reshaping human antibodies for therapy. Nature 1988; 332: 323–327.
Dyer MJ, Hale G, Hayhoe FG, Waldmann H . Effects of CAMPATH-1 H antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood 1989; 73: 1431–1439.
Greenwood J, Clark M, Waldmann H . Structural motifs involved in human IgG antibody effector functions. Eur J Immunol 1993; 23: 1098–1104.
Gilleece MH, Dexter TM . Effect of Campath-1 H antibody on human hematopoietic progenitors in vitro. Blood 1993; 82: 807–812.
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87: 4990–4997.
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 13–47.
Döhner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1910–1918.
Kröber A, Seiler T, Benner A, Bullinger L, Bruckle E, Lichter P et al. VH mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood 2002; 100: 1410–1416.
Bruggemann M, Droese J, Bolz I, Luth P, Pott C, von Neuhoff N et al. Improved assessment of minimal residual disease in B cell malignancies using fluorogenic consensus probes for real-time quantitative PCR. Leukemia 2000; 14: 1419–1425.
Kern F, Surel IP, Brock C, Freistedt B, Radtke H, Scheffold A et al. T-cell epitope mapping by flow cytometry. Nat Med 1998; 4: 975–978.
Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ . Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest 1997; 99: 1739–1750.
Longmate J, York J, La Rosa C, Krishnan R, Zhang M, Senitzer D et al. Population coverage by HLA class-I restricted cytotoxic T-lymphocyte epitopes. Immunogenetics 2001; 52: 165–173.
Kaplan E, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Greenwood M . The natural duration of cancer. Reports on Public Health and Medical Subjects. London, UK: Her Majesty's Stationary Office, 1926, pp 1–26.
Anderson JR, Cain KC, Gelber RD . Analysis of survival by tumor response. J Clin Oncol 1983; 1: 710–719.
Hertenstein B, Wagner B, Bunjes D, Duncker C, Raghavachar A, Arnold R et al. Emergence of CD52-, phosphatidylinositolglycan-anchor-deficient T lymphocytes after in vivo application of Campath-1 H for refractory B-cell Non-hodgkin lymphoma. Blood 1995; 86: 1487–1492.
Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of CAMPATH-1 H in delaying immune reconstitution. Blood 2002; 99: 4357–4363.
Nguyen DD, Cao TM, Dugan K, Starcher SA, Fechter RL, Coutre SE . Cytomegalovirus viremia during CAMPATH-1 H therapy for relapsed and refractory chronic lymphocytic leukemia and prolymphocytic leukemia. Clin Lymphoma 2002; 3: 105–110.
Montillo M, Cafro AM, Tedeschi A, Brando B, Oreste P, Veronese S et al. Safety and efficacy of subcutaneous Campath-1 H for treating residual disease in patients with chronic lymphocytic leukemia responding to fludarabine. Haematologica 2002; 87: 695–700.
Morra E, Nosari A, Montillo M . Infectious complications in chronic lymphocytic leukaemia. Hematol Cell Ther 1999; 41: 145–151.
O'Brien SM, Kantarjian HM, Thomas DA, Cortes J, Giles FJ, Wierda WG et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer 2003; 98: 2657–2663.
Taylor VC, Sims M, Brett S, Field MC . Antibody selection against CD52 produces a paroxysmal nocturnal haemoglobinuria phenotype in human lymphocytes by a novel mechanism. Biochem J 1997; 322: 919–925.
Rawstron AC, Rollinson SJ, Richards S, Short MA, English A, Morgan GJ et al. The PNH phenotype cells that emerge in most patients after CAMPATH-1 H therapy are present prior to treatment. Br J Haematol 1999; 107: 148–153.
Florena AM, Iannitto E, Quintini G, Franco V . Bone marrow biopsy in hemophagocytic syndrome. Virchows Arch 2002; 441: 335–344.
Enblad G, Hagberg H, Erlanson M, Lundin J, Porwit Mac Donald A, Repp R et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood 2003, [Epub ahead of print] Dec 30, 2003.
Garg M, Moore H, Tobal K, Lin Yin JA . Prognostic significance of quantitative analysis of WT1 gene transcripts by competitive reverse transcription polymerase chain reaction in acute leukaemia. Br J Haematol 2003; 123: 49–59.
Paschka P, Müller MC, Merx K, Kreil S, Schoch C, Lahaye T et al. Molecular monitoring of response to imatinib (Glivec) in CML patients pretreated with interferon alpha. Low levels of residual disease are associated with continuous remission. Leukemia 2003; 17: 1687–1694.
Scheuring UJ, Pfeifer H, Wassmann B, Bruck P, Gehrke B, Petershofen EK et al. Serial minimal residual disease (MRD) analysis as a predictor of response duration in Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL) during imatinib treatment. Leukemia 2003; 17: 1700–1706.
Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G . Pharmacokinetics of CAMPATH-1 H in BMT patients. Cytotherapy 2001; 3: 261–267.
Rebello P, Hale G . Pharmocokinetics of CAMPATH-1H: assay development and validation. J Immunol Methods 2002; 260: 285–302.
Hainswirth JD, Litchy S, Barton JH, Houston GA, Hermann RC, Bradof JE et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2003; 21: 1746–1751.
Acknowledgements
We are grateful to the following colleagues taking care of patients being enrolled on study protocol: Dr Abenhardt, Munich; PD Dr Benz, Tübingen; Professor Dr Dührsen, Essen; Dr Fiedler, Chemnitz; Dr Freier, Hildesheim; Dr Hopfinger, Wien; Dr Kamp, Wendlingen; Dr Lambertz, Garmisch-Partenkirchen; Dr Lindemann, Hagen; Dr Middecke, Lemgo; PD Dr Mittermüller, Munich; Dr Pasold, Potsdam; Dr Srock, Berlin; Dr Stauch, Kronach; Dr Ziegler, Erlangen. We appreciate especially the support of D. Egger and Dr Grassmann, Medac-Schering Onkologie GmbH, and Dr Kuhlmann, Schering AG. We thank Dr Hillmen and Dr Hale for inspiring discussions. This study was supported by a grant from Schering AG and ILEX Oncology.
Author information
Authors and Affiliations
Consortia
Additional information
Presented, in part, at the 39th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31–June 3, 2003, and the 45th Annual Meeting of the American Society of Hematology, San Diego, CA, December 6–9, 2003.
Supported by a research grant of Schering AG, Berlin and MedacSchering Onkologie, Germany. VH sequencing was supported by a research grant from the Else Kröner-Fresenius Stiftung, Germany.
Rights and permissions
About this article
Cite this article
Wendtner, CM., Ritgen, M., Schweighofer, C. et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission – experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 18, 1093–1101 (2004). https://doi.org/10.1038/sj.leu.2403354
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403354
Keywords
This article is cited by
-
The impact of T-cell depletion techniques on the outcome after haploidentical hematopoietic SCT
Bone Marrow Transplantation (2014)
-
Rapid generation of human B-cell lymphomas via combined expression of Myc and Bcl2 and their use as a preclinical model for biological therapies
Oncogene (2013)
-
Chronic Lymphocytic Leukemia: Inception to Cure: Are We There?
Indian Journal of Hematology and Blood Transfusion (2013)
-
What Do We Do with Chronic Lymphocytic Leukemia with 17p Deletion?
Current Hematologic Malignancy Reports (2013)
-
Can Prognostic Factors Be Used to Direct Therapy in Chronic Lymphocytic Leukemia?
Current Hematologic Malignancy Reports (2012)