Abstract
Background:
The metabolic implications of intermuscular adipose tissue (IMAT) are poorly understood compared to those of visceral adipose tissue (VAT) even though the absolute quantities of both depots are similar in many individuals.
Objective:
The aim was to determine the independent relationship between whole-body IMAT and cardiovascular risk factor parameters.
Design:
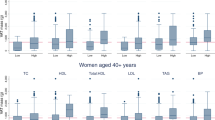
Whole body magnetic resonance imaging (MRI) was used to quantify total skeletal muscle (SM), total adipose tissue (TAT) of which IMAT, defined as the AT visible by MRI within the boundary of the muscle fascia, is a sub-component. Fasting serum measures (n=262) of glucose, total cholesterol (T-Chol), high-density lipoprotein cholesterol (HDL-Chol), triglycerides (TG), protein bound glucose (PBG, n=206) and insulin (n=119) were acquired in healthy African-American (AA, n=78) and Caucasian (Ca, n=109) women (body mass index (BMI) 26.5±5.7 kg/m2; 44.4±16.4 years) and men (39 AA, 62 Ca; BMI 25.6±3.5 kg/m2; 45.6±17.4 years). General linear models identified the independent effects of IMAT after covarying for SM, VAT, TAT, race, sex and two-way interactions.
Results:
Significant independent associations were observed for IMAT with glucose (P<0.001), PBG (P<0.001) and T-Chol (P<0.05). The association of IMAT with cholesterol differed by race in such a manner that for a unit increase in IMAT, T-Chol increased more rapidly in Ca compared to AA (P<0.05). TG, HDL-Chol and insulin had no independent association with IMAT.
Conclusion:
The strong independent associations of IMAT with fasting glucose and PBG suggest that IMAT may be related to glucose metabolism; however, IMAT is also associated with T-Chol in Ca.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boden G . Obesity, free fatty acids, and insulin resistance. Cur Opin Endo Diabetes 2001; 8: 235–239.
Hotamisligil GS, Shargill NS, Spiegelman BM . Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–946.
Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ et al. Abdominal adiposity and coronary heart disease in women. JAMA 1998; 280: 1843–1848.
Kannel WB, Cupples LA, Ramaswami R, Stokes J III, Kreger BE, Higgins M . Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol 1991; 44: 183–190.
Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254–260.
Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L . Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ 1984; 289: 1257–1261.
Han TS, van Leer EM, Seidell JC, Lean ME . Waist circumference as a screening tool for cardiovascular risk factors: evaluation of receiver operating characteristics (ROC). Obes Res 1996; 6: 533–547.
Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L et al. The influence of body fat distribution on the incidence of diabetes mellitus 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985; 34: 1055–1058.
Seidell JC, Han TS, Feskens EJ, Lean ME . Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med 1997; 242: 401–406.
Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM . Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endo Meta 2005; 90: 4573–4578.
Kahn HS, Austin H, Williamson DF, Arensberg D . Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol 1996; 49: 1017–1024.
Tan GD, Goossens GH, Humphreys SM, Vidal H, Karpe F . Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obesity Res 2004; 12: 114–118.
Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005; 165: 777–783.
Goodpaster BH, Thaete FL, Kelley DE . Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885–892.
Albu JB, Engelson ES, He Q, Berk E, Kotler DP . Upper body subcutaneous adipose tissue distribution and increased inter-muscular adipose (IMAT) tissue independently predict insulin resistance (IR) in HIV-infected obese women. Obesity Res 2005; 13 (Suppl): A48 (Abstract).
Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D . Sarcopenia and increased muscle adipose tissue infiltration in elderly African-American women. Am J Clin Nutr 2004; 79: 874–880.
Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005; 81: 903–910.
Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 2005; 82: 1010–1017.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE . Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579–1585.
Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003; 26: 372–379.
Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH . Report of the Task Group on Reference Men. International Commission on Radiological Protection no. 23. Pergamon Press: Oxford, United Kingdom, 1975.
O'Brien JE, Brookes M . Determination of reference values for a novel ketoamine-specific fructosamine assay for assessment of diabetic glycemic control. Diabetes Technol Ther 1999; 1: 457–459.
Maggs DG, Jacob R, Rife F, Lange R, Leone P, During MJ et al. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadricep muscle and adipose tissue. Evidence for significant lipolysis in skeletal muscle. J Clin Invest 1995; 96: 370–377.
Ryan AS, Nicklas BJ . Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord 1999; 23: 126–132.
Lamon-Fava S, Wilson PW, Schaefer EJ . Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring study. Arterioscler Thromb Vasc Biol 1996; 16: 1509–1515.
Lemieux S, Prud'homme D, Moorjani S, Tremblay A, Bouchard C, Lupien PJ et al. Do elevated levels of abdominal visceral adipose tissue contribute to age-related difference in serum lipoprotein concentrations in men? Atherosclerosis 1995; 118: 155–164.
Miyawaki T, Abe M, Yahata K, Kajiyama N, Katsuma H, Saito N . Contribution of visceral fat accumulation to the risk factors for atherosclerosis in non-obese Japanese. Inter Med 2004; 43: 1138–1144.
Jensen MD . Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity 2006; 14: 20S–24S.
Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX . Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol 1999; 277: E552–E560.
Matsuzawa Y . Pathophysiology and molecular mechanisms of visceral fat syndrome: The Japanese experience. Diabetes Metab Rev 1997; 13: 3–13.
Bjorntorp P . “Portal”. adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990; 10: 493–496.
Bertin E, Nguyen P, Guenounou M, Durlach V, Potron G, Leutenegger M . Plasma levels of tumor necrosis factor-alpha (TNF-α) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab 2000; 26: 178–182.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001; 86: 1930–1935.
Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48: 301–308.
Janssen I, Fortier A, Hudson R, Ross R . Effects of an energy-restrictive diet with or without exercise on abdominal fat, inter-muscular fat, and metabolic risk factors in obese women. Diabetes Care 2002; 25: 431–438.
Acknowledgements
We acknowledge the contributions of Mark Punyanitya, the Director of Image Reading Center where MRI analyses were performed and Dr Blandine Laferrere and Dr F Xavier Pi-Sunyer for their critical input.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH AG14715, DK42618; RR00645, DK40414; and a contract from the NIA.
Rights and permissions
About this article
Cite this article
Yim, JE., Heshka, S., Albu, J. et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes 31, 1400–1405 (2007). https://doi.org/10.1038/sj.ijo.0803621
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803621
Keywords
This article is cited by
-
The association of body composition and fat distribution with dysmobility syndrome in community-dwelling older adults: Bushehr Elderly Health (BEH) program
BMC Musculoskeletal Disorders (2023)
-
Abdominal fat and muscle distributions in different stages of colorectal cancer
BMC Cancer (2023)
-
Intermuscular adipose tissue in metabolic disease
Nature Reviews Endocrinology (2023)
-
Neck circumference is associated with adipose tissue content in thigh skeletal muscle in overweight and obese premenopausal women
Scientific Reports (2020)
-
Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss
GeroScience (2020)