Abstract
This study, based on morphological and isozyme analysis, clearly discriminates two invasive Carpobrotus taxa, C. edulis and C. acinaciformis, in the Hyères archipelago off the southeastern coast of France. However, three different allelic combinations demonstrate the presence of intermediate individuals resulting from an introgression of part of the C. edulis genome into that of C. acinaciformis. Both taxa have higher than average genetic (C. edulis: P(0.95)=62.5%, A=2.25±0.70, Ho=0.329±0.324; C. acinaciformis: P(0.95)=75%, A=2.38±0.42, Ho=0.645±0.109) and clonal diversities (C. edulis: IP=0.37; C. acinaciformis: IP=0.48). Furthermore, C. acinaciformis has an excess of heterozygotes (F=−0.585±0.217), probably due to introgression. The relationship between the probability of clonal identity for two individuals and distance indicates that C. acinaciformis relies more on clonal reproduction than on sexual recruitment (seed recruitment/vegetative propagation=u/v=0.027), in contrast to C. edulis, whose probability of clonal identity did not vary with distance. The overwhelming clonal growth and high genetic diversities of C. acinaciformis and the previously recorded invasion potential for C. edulis raises concern for intensified invasion via hybridisation.
Similar content being viewed by others
Introduction
Invasive species have become a growing global concern (D'Antonio and Dudley, 1995; Vitousek et al, 1996; Ewel et al, 1999), and are now regarded as the second-most important threat to the maintenance of biodiversity, after the fragmentation and/or destruction of habitats (Williamson, 1996). Invasions often modify population dynamics, community structure, the composition and functioning of ecosystems, and may accelerate the extinction of indigenous species (Macdonald et al, 1988; Higgins et al, 1996; Vitousek et al, 1996; Williamson, 1996; Daehler, 1998).
In this study, we examine the invasion of two alien Carpobrotus (Aizoaceae) taxa purportedly present on islands in the Mediterranean Basin. C. edulis (L.) N. E. Br. and C. acinaciformis (L.) L. Bol were introduced into southeastern France in the early 1800s (Gouffé de la Cour, 1813) and have since become naturalised along the coastline and on the Hyères archipelago (Albert & Jahandiez, 1908). C. edulis, due to its higher occurrence, is considered to be more invasive than C. acinaciformis, and as a severe threat to several rare or endangered native plant species (Lambinon, 1995; Suehs et al, 2001). However, recent taxonomic investigation has brought into question just exactly which Carpobrotus taxon is present in the Mediterranean Basin and is the most aggressive towards the indigenous flora. In contradiction to several decades of recording, the presence of two or more species of Carpobrotus, Akeroyd and Preston (1990), (1993), basing their observations on foliar characteristics, concluded that C. edulis is the only species present in the Mediterranean Basin, although in the form of several varieties with different flower colours. Further complicating the situation is the possibility of hybridisation between Carpobrotus species, which has been clearly demonstrated between C. edulis and C. chilensis in California (Albert et al, 1997; Gallagher et al, 1997), and noted between C. edulis and three other species, including C. acinaciformis in South Africa (Wisura and Glen, 1993) and C. virescens in Australia (Blake, 1969).
The implications of hybridisation are of great importance since reports on hybridisation between native and alien species and/or between aliens are increasing, particularly in island habitats (Abbott, 1992), and may result in negative genetic and ecological impacts on native plant species (Vilà et al, 2000). Natural hybrids vary greatly in their viability and fertility, and in general, have a rapid response to natural selection (Rieseberg and Carney, 1998). This can lead, via backcrossing and advanced generation hybrids, to (i) an equilibrated coexistence of the two parental species and a hybrid zone, or (ii) introgressions affecting the relative fitnesses of parental species and/or hybrids. The first case maintains a sort of status quo in which the small amount of gene flow that does occur between the two parental species is not enough to break down specific distinctions (Barton and Hewitt, 1985, and 1989; Carney et al, 2000), and is therefore unlikely to affect invasion processes. However, the second case can result in hybrid speciation due to polyploidisation (Abbott, 1992), niche separation between parental species and hybrid taxa (Arnold, 1992; Rieseberg, 1997), or reproductive isolation as a by-product of fertility selection (Rieseberg, 2000). It can also produce the reduction or extinction of one or both parental species to the advantage of a hybrid swarm presenting genotypic combinations advantageous in the invaded habitat (Carney et al, 2000). These more dramatic instances of taxonomic rearrangement are likely to greatly affect invasion processes.
In light of the above-mentioned factors, we are initiating a series of ecological and evolutionary studies in order to better understand the invasion of Carpobrotus taxa along the Provencal coast. Using morphometric and allozyme analyses, we evaluate two distinct populations of Carpobrotus representing different potential genetic backgrounds on the island of Bagaud, a highly invaded, ‘off-limits’ reserve within the French National Park of Port-Cros. We specifically address the following questions: (1) how do gentoypes compare between the purported C. edulis and C. acinaciformis individuals, and are the genotypic and morphological markers consistent with the idea that these are discrete lineages within Carpobrotus; (2) what is the potential for and the possible implications of hybridisation between taxa; and (3) what differences exist between taxa at the genetic and clonal diversity and structure levels?
Materials and methods
Study taxa and sites
C. edulis (L.) N. E. Br. and C. acinaciformis (L.) L. Bolus (Aizoaceae) are perennial, clonal, trailing succulents characterised by a mat-forming habit and thick, three-dimensional, elongated leaves with a triangular cross-section. Flowers are solitary and produce fleshy, indehiscent fruits whose seeds are dispersed by endozoochory (Vilà and D'Antonio, 1998a). Within their native range of South Africa, the two taxa can be visually distinguished by flower colour: C. edulis is the only member of its genus having distinctly yellow flowers fading to light pink, while C. acinaciformis has vivid magenta flowers (Wisura and Glen, 1993). This distinction has however, been challenged by Akeroyd and Preston (1990), (1993), who claim that no morphological differences exist between plants with different coloured flowers in the Mediterranean Basin, and therefore they should be grouped as varieties under one species: C. edulis.
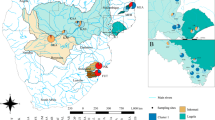
In the Mediterranean Basin, magenta and yellow flowered plants are present on the coastline, and found in a variety of habitats: cliffs, beaches, maquis and rock outcrops (Suehs et al, 2001). On the islands of Hyères, off the Provencal coast of France, both taxa are very prevalent, and the largest mats are found on the island of Bagaud (a 45 ha island reserve within the French National Park of Port-Cros), which is located 7500 m from the continental coast and 450 m west of the island of Port Cros (Figure 1). The island is completely off-limits to human visitation, which ensures that the invasion processes studied are as ‘natural’ as possible. Bedrock consists of quartzites, micaschists, and phyllites, and the climate is subhumid to humid within the thermomediterranean bioclimate (Médail, 1998).
Two populations of Carpobrotus, at least 250 m distant from each other, were selected on the island of Bagaud. The remaining populations are inaccessible. The first population (Population 1; Figure 1) consists for the most part of purported C. acinaciformis, having magenta flowers, with some yellow-flowered clusters mixed in. It is located on the southwestern part of the island, at a mean altitude of 42 m above sea level. This population, which covers about 1500 m2, overlaps the interior of the island where it is colonising the low, coastal matorral. The second population (Population 2; Figure 1) is composed entirely of purported C. edulis, having only yellow flowers. It is located on the eastern side of the island, at a mean altitude of 5 m. This 2050 m2 population has developed on the coastal belt, rich in halophilic and haloresistant species.
Leaf sampling, protein extraction, and electrophoresis
In order to locate different individuals within multiclonal mats spatially, leaves were sampled using a 3 × 3 m grid. A few individuals from small ‘satellite’ patches, indicative of a sexual mode of reproduction, were incorporated into the grid system. In January and February 1999, four to five young leaves were harvested at each grid point, totalling 265 samples from Population 1 and 268 samples from Population 2. Samples were kept at 4°C until the time of analysis.
Leaf material was ground with an extraction modified from Wendel and Weeden (1990): 0.1 M Tris-HCl buffer, pH 7.5; 10% polyvinylpyrrolidone MW 40 000; 0.25 M ascorbic acid, Na salt; 0.02 M diethyldithiocarbamate; 1% bovine albumin; 0.2 M sodium borate. Extracts were then centrifuged for 20 min at 15 000 g. The resulting crude protein extracts were absorbed on 3 mm × 15 mm paper wicks and loaded into 12.5% starch gels for enzyme electrophoresis. Extraction and electrophoresis stages were performed either on ice or in a refrigerated chamber at 4°C.
Among 13 essayed enzyme systems, seven demonstrated scorable results: acid phosphatase (ACP; E.C. 3.1.3.2), malate dehydrogenase (MDH; E.C. 1.1.1.37), 6-phosphogluconate dehydrogenase (PGD; E.C. 1.1.1.44), phosphoglucoisomerase (PGI; E.C. 5.3.1.9), phosphoglucomutase (PGM; E.C. 5.4.2.2), shikimate dehydrogenase (SKD; E.C. 1.1.1.25), and isocitrate dehydrogenase (IDH; E.C. 1.1.1.42). Eight scorable loci for each taxon were visualised using the gel and electrode buffer systems detailed in Table 1. Staining procedures followed Wendel and Weeden (1990). Band patterns for progeny resulting from controlled crosses were consistent with diploid inheritance for both taxa, which was supported by karyotyping (2n=2x=18: Verlaque, in Médail, 1999). Loci and alleles were numbered with integers from slowest to fastest, and all calculations were carried out on both the ramet and genet levels in order to detect the differences arising from the potential pseudoreplication of clones (Cook, 1983). Allelic combinations were used to distinguish genotypically different individuals for use in morphometric analysis.
Genetic diversity and structure
Five indicators of genetic diversity were calculated using GENETIX 4.0 (Belkhir, 1999) for each population: allelic frequencies for each locus, the percentage of polymorphic loci (P; 0.95 criterion), the mean number of alleles per locus (A), and the mean observed (Ho) and expected heterozygoty (He) under Hardy–Weinberg equilibrium. The GENEPOP population software package (Raymond and Rousset, 1995) was used to explore the genetic structure within and between populations. Wright's (1965) inbreeding coefficient (F) was used to quantify the differences between observed heterozygosity and Hardy–Weinberg equilibrium; (Weir and Cockerham, 1984). Probability-exact tests were used to test Hardy–Weinberg equilibrium. Between-population differentiation was quantified using FST, (Wright, 1965; Weir and Cockerham, 1984), and tested using genic and genotypic exact tests. Nei's (1973), (1977) genetic distance was calculated between C. edulis, C. acinaciformis, and between subpopulations using GENETIX 4.0 (Belkhir, 1999).
Clonal diversity and recruitment by vegetative propagation
In order to evaluate clonal diversity, the identifiable proportion (IP) of clones (number of clones/number of individuals) was calculated according to Ellstrand and Roose (1987). We analysed the ratio of recruitment by seed to recruitment by vegetative propagation (u/v) using the probability of clonal identity F(r). The parameter u/v is determined according to the following equations:  , where k is a constant, r represents distance, and σ2 is the squared mean distance between ramets (Harada et al, 1997). F(r) and r are calculated and plotted against each other in order to show how the probability of clonal identity varies as a function of sampling distance. Having calculated σ2, maximum-likelihood estimates of the two parameters k and c are calculated by adjusting F(r) and r to the above-mentioned equations using S-PLUS software (MathSoft, 1999). Distance ranges (m) used in this study were determined by integers, and we chose to eliminate those distance ranges consisting of fewer than 30 pairs of individuals from the model in order to avoid basing an estimate of F(r) for a particular distance on an insufficient number of samples (Sokal and Rohlf, 1995).
, where k is a constant, r represents distance, and σ2 is the squared mean distance between ramets (Harada et al, 1997). F(r) and r are calculated and plotted against each other in order to show how the probability of clonal identity varies as a function of sampling distance. Having calculated σ2, maximum-likelihood estimates of the two parameters k and c are calculated by adjusting F(r) and r to the above-mentioned equations using S-PLUS software (MathSoft, 1999). Distance ranges (m) used in this study were determined by integers, and we chose to eliminate those distance ranges consisting of fewer than 30 pairs of individuals from the model in order to avoid basing an estimate of F(r) for a particular distance on an insufficient number of samples (Sokal and Rohlf, 1995).
Comparative morphology
An analysis of 14 foliar and reproductive characters was carried out to discriminate Carpobrotus taxa phenotypically. During the flowering and fruiting seasons of 1999, 37 and 32 clones identified through electrophoresis were selected in Populations 1 and 2, respectively. For each clone, basal, mature leaves, flowers, and fruits on flowering branchlets were subjected to the following measures (Figure 2): the number of carpels per fruit (NC), stamen ring diameter (SRD; mm), receptacle width (RW; mm), receptacle length (RL; mm), the average leaf length (LL; mm), the width (W1; mm) and thickness (T1; mm) of leaf cross-sections at 3 cm from the point of insertion, and the width (W2; mm) and thickness (T2; mm) of leaf cross-sections at 1 cm from the leaf apex. The internode length (IL; mm) between flowering branchlets was also measured. Three replicates were averaged per clone, with the exception of leaf lengths where six replicates per clone were used. An equilaterality index for each cross-sectional triangle was calculated in the following manner: E1=W1/T1 and E2=W2/T2. The ratio of leaf width over leaf length (W1/LL) and the difference E1–E2 were also quantified. Measures were taken using callipers, precise to 0.1 mm.
Flowering branchlet of Carpobrotus demonstrating morphometric characters. The number of stamens has been reduced, petals removed, and the bud drawn in cross-section for clarity. Figure abbreviations: SRD, stamen ring diameter; NC, number of carpels; W1, width 1; W2, width 2; T1, thickness 1; T2, thickness 2; LL, leaf length; RW, receptacle width; RL, receptacle length.
The means and standard deviations for each foliar or reproductive character were then calculated for each of the two populations. A discriminate analysis was performed to test the differences between Populations 1 and 2 for all characteristics. The standard canonical coefficients for each character on each significant canonical axis were calculated to determine the contribution of each character to the taxon discrimination. Measured characters were log transformed when necessary in order to minimise heteroscedasticity (Sokal and Rohlf, 1995). All morphometric statistical analyses were performed using STATISTICA 5.1 software (StatSoft France, 1997).
Results
Loci descriptions and hybrid characterisation
Of the 265 and 268 samples taken from Populations 1 and 2 respectively, only 253 from each provided usable extracts for protein analysis. The seven systems analysed provided eight scorable loci for Population 1 (Acp-1, Pgd-1, Pgi-1, Pgm-1, Pgm-2, Skd-1, Idh-1 and Idh-2) and Population 2 (Acp-1, Pgd-1, Pgi-1, Skd-1, Mdh-1, Mdh-2, Idh-1, and Idh-2). Acp-1, Mdh-2, Pgd-1, and Pgi-1 had banding patterns consistent with a dimeric enzyme, while Mdh-1, Pgm-1, Pgm-2, and Skd-1 were monomeric. Only one allozyme was found for Idh-1 and Idh-2 in Populations 1 and 2, and Skd-1 in Population 2, and therefore these loci were considered monomorphic. Population 1 also had an Mdh-1 locus. However, this locus was used only in hybrid identification and not included in further genetic analyses due to duplication. Among the eight scorable loci, six were polymorphic in Population 1 and five in Population 2.
Isozymes are considered neutral to selection (de Vienne, 1998), and therefore a valid measure of population differentiation. Alleles or allele combinations were characteristic of one population or the other, and used as taxon markers (Maki and Murata, 2001). Population 1 was characterised by: (1) the presence of allele 1 at Skd-1; (2) the absence of locus Mdh-2; and (3) the presence of allele 3 at Acp-1 (Table 2). The genetic characteristics of Population 2 were: (1) the fixation of allele 2 at Skd-1; (2) the existence of locus Mdh-2; and (3) the presence of allele 2 at Acp-1 (Table 2). Those individuals demonstrating a mixture of these characteristics were considered as intermediate types, and were only detected in Population 1. Thus, an ‘intermediate type’ is an individual demonstrating any combination of the above-mentioned characters, and not necessarily a 50/50 hybrid between the two populations.
Thus, among the 253 samples representing Population 1, 84 were determined to be C. acinaciformis and 169 to be intermediate types. We use these two groups as sub populations of Population 1, the totality of which we re-designate as a ‘hybrid swarm.’ The 253 samples representing Population 2 consisted of C. edulis.
Genetic and clonal diversity
Allele frequencies for the subpopulations, C. acinaciformis and intermediate types, plus the hybrid swarm and C. edulis on the ramet and genet levels are detailed in Table 2. Allele frequencies at the ramet and genet levels, taking into account all polymorphic loci, differed by 6.27%. This difference was mostly due to the increased importance of rare alleles that coincides with the elimination of ramet repetition necessary for shifting to the genet level.
The change from the ramet to genet levels does not affect the percentage of polymorphic loci or the number of alleles per locus. The percentage of polymorphic loci is greater for C. acinaciformis (75.0%), the intermediate types (75.0%), and the hybrid swarm (75.0%) compared to C. edulis (62.5%). In a similar manner, the average number of alleles per locus was highest for the intermediates (2.88±0.69), the hybrid swarm (2.88±0.69), and to a lesser degree C. acinaciformis (2.38±0.42), when compared to C. edulis (2.25±0.70).
For Population 2, differences at the ramet and genet levels between the observed and expected heterozygosities are very weak, in contrast to Population 1, where the observed heterozygosities are systematically higher than expected heterozygosity values.
Population 1 contained 121 clones (Figure 3a), that is, an IP of 0.48. Of these, 34 were C. acinaciformis with an IP of 0.40, and 87 were intermediate types with an IP of 0.52. For Population 2, 93 genotypes were found (Figure 3b), with a corresponding IP of 0.37.
Genetic and clonal structure
The average fixation indices (F) at the ramet and genet levels were quite negative for Population 1, while fixation index values are close to zero for Population 2 (Table 3). Although two loci for C. acinaciformis tested nonsignificant for the Hardy–Weinberg probability exact test, the remaining loci in all populations, and all Hardy–Weinberg probability tests at the multilocus level were significant (Table 3). The subpopulations C. acinaciformis and intermediate types had very weak multilocus FST values at both the ramet (0.009) and genet (0.001) levels, respectively. Allelic and genotypic distributions across the two subpopulations were significantly different at the ramet level (χ2=53.71, d.f.=12, P<0.001 and χ2=∞, d.f.=12, P<0.001, respectively) according to population differentiation exact tests. However, at the genet level, these same two tests were nonsignificant. Nei's genetic distance (D) between C. acinaciformis and C. edulis is higher (0.153) than the distances between C. edulis and the intermediate types (0.119). The former is also higher, and to a greater extent, than the genetic distance between C. acinaciformis and the intermediate types (0.005).
The relationships between the probability of clonal identity F(r) and distance (m) were quite different for both populations (Figure 4). For Population 1, F(r) decreases logarithmically with distance. The parameter σ2 (152.67) allowed the estimation of c (0.019±0.015; t=21.99) and k (0.329±0.002; t=7.97), with a ratio of u/v of 0.027. For Population 2, the probability of clonal identity did not appear to vary with distance. The parameter σ2 (2235.40) allowed the estimation of c (−0.009±0.012; t=12.15) and k (0.014±0.001; t=−7.47), with a ratio of u/v of 0.090.
Comparative morphology
The clones used in the morphological study represent each population in its entirety without regard to the previous subpopulation divisions in Population 1. The mean values and standard deviations for all characters in both populations are shown in Figure 5. The discriminate analysis results indicated the existence of significant (F=37.43; d.f.=14, 53; P<0.001) differences between the two populations over all morphometric characters. All individuals were correctly classified as Population 1 or Population 2. The standardised canonical coefficients for leaf length (1.72), width 1/leaf length (1.50), width 1 (−1.15), and to a lesser degree E1–E2 (0.89), and internode length (−0.71) all permitted a clear discrimination of the two populations on the canonical axis.
Means and S.D.'s for 14 morphometric characters measured on genetically distinct individuals for Population 1 (C. acinaciformis and intermediate types, the totality of which forms a hybrid swarm) and Population 2 (C. edulis). The number of individuals available for analysis is given in parentheses. Figure abbreviations: NC, number of carpels; SRD, stamen ring diameter (mm); IL, internode length (mm); LL, leaf length (mm); W1, width 1 (mm); T1, thickness 1 (mm); E1, equilaterality index 1; W2, width 2 (mm); T2, thickness 2 (mm); E2, equilaterality index 2; RW, receptacle width (mm); RL, receptacle length (mm).
Discussion
Morphometric and genetic characterisation of Carpobrotus taxa
The present study based on morphometric and genetic variation clearly indicates distinct taxonomic origins for Populations 1 and 2. The reproductive and foliar traits used in this study are consistent with qualitative and quantitative descriptions only for C. acinaciformis and C. edulis in several floras (Tutin et al, 1964; Blake, 1969; Fernandes, 1972; Haslam et al, 1977; Huxley and Taylor, 1977; Wisura and Glen, 1993), and the relative genetic distances that we have found between C. edulis, C. acinaciformis, and intermediate groups also support the existence of two distinct taxa. This contradicts the findings of Akeroyd and Preston (1990), (1993), stating that only one taxon is present and that citations for C. acinaciformis in the Mediterranean Basin are misidentifications of a magenta-flowered C. edulis (var. rubescens Druce). We therefore confirm the presence of two taxa on the island of Bagaud and designate Population 1 as C. acinaciformis and Population 2 as C. edulis.
Similar morphological and genetic studies have been carried out in California with supporting results (Albert et al, 1997). Among the six characters in common with the present study, three foliar characteristics discriminated C. edulis and C. chilensis in California and C. edulis and C. acinaciformis in Provence: leaf length, leaf width, and leaf width/leaf length. The present study also indicates that internode lengths and leaf equilaterality indices are also useful for discriminating Carpobrotus taxa. Furthermore, the value of Nei's (1987) genetic identity reported for C. edulis and C. chilensis in California corresponds to a genetic distance of 0.13 (Gallagher et al, 1997), which is quite similar to that found between C. edulis and C. acinaciformis (0.15) in the present study. C. edulis in California also shares certain allele frequencies with C. edulis in Provence, such as the fixation of one allele at Idh 1, Idh 2, and Skd loci.
The second important feature of the present study is the existence of intermediate types in Population 1. The fact that C. acinaciformis maximises seed production via hybridisation with C. edulis (Suehs et al, in press) strongly supports that hybridisation occurs in situ on the island of Bagaud, and helps to explain the preponderance of intermediate types. It is also possible that introgressed individuals were involved in the original colonisation process, and we have analysed their descendants. We consider the totality of this population to form a hybrid swarm, that is, ‘a complex mixture of parental forms, F1 hybrids, backcross types, and segregation products (Grant, 1971)’. The large number of weakly differentiated intermediate types relative to C. acinaciformis suggests introgression of the C. edulis genome into that of C. acinaciformis. This recurrent introgression is probably produced via backcrosses more frequent in relation to recombinations of subsequent generations (Rieseberg and Carney, 1998).
Genetic and clonal diversity
Life history traits are recognised as major factors having the capacity to influence the level of genetic diversity and its organisation within and between populations of a large range of plant species (Hamrick et al, 1979). In each of our subpopulations and populations (C. edulis, C. acinaciformis, the diverse intermediate types and the totality of the hybrid swarm), we found levels of genetic diversity (percentage of polymorphic loci, mean number of alleles per locus, and observed heterozygosity) higher than the averages for species with asexual, sexual, allogamous, mixed modes of reproduction or a zoochoric seed dispersal mechanism (Hamrick et al, 1979). C. acinaciformis, the diverse intermediate types and the entire hybrid swarm also had higher genetic diversity means than long-lived, perennial species. These high levels of genetic diversity are also supported by the identifiable proportion of clones, which are higher than the average cited in Ellstrand and Roose (1987) for 21 clonal species (0.17). Thus, in spite of the fact that both taxa are clonal, they also successfully reproduce by sexual means, with C. acinaciformis and the intermediate types expressing higher genetic diversities than C. edulis.
The levels of genetic diversity for C. edulis and the diverse hybrids C. edulis × C. chilensis in coastal California are higher than those of C. chilensis (Gallagher et al, 1997). The C. edulis reported in California appears to be introduced as a hybrid taxa receiving gene flow from C. chilensis (Gallagher et al, 1997). Similar to the situation of C. edulis and C. chilensis in California, this study suggests for the first time that C. acinaciformis on the island of Bagaud could be a hybrid introduced taxon that received an introgressive flux from a relatively purer parent, C. edulis. In fact, intermediate types outnumber parental genotypes in Population 1, which supports a largely hybrid origin for C. acinaciformis on Bagaud island, where a sustained introgression has taken place.
Genetic and clonal structure
Wright's average inbreeding coefficients (F) for C. edulis are quite close to zero, which suggests that this population is very close to Hardy–Weinberg equilibrium. In C. acinaciformis, the diverse intermediate types and the hybrid swarm, the relatively high, negative average Wright's inbreeding coefficient (F) suggests a high frequency of gene flow in free pollination between nonrelated individuals, and therefore little inbreeding in C. acinaciformis and the diverse intermediate types. This is supported by Hardy–Weinberg, probability-exact tests at the multilocus level, all of which indicate an excess of heterozygotes. However, intermediate types differ only slightly from C. acinaciformis individuals as indicated by the extremely weak FST values, and also by nonsignificant population differentiation at the genet level. This suggests that hybridisation has been occurring on Bagaud long enough to allow tangible amounts of backcrossing.
Since the spatial distribution of genotypes is most often autocorrelated, there should be a negative relationship between the distance separating two individuals and the probability that they belong to the same clone (Harada et al, 1997). C. acinaciformis shows such a relationship, and the standard deviation of the average distance between individuals having the same genotype is 12.4 m, which indicates the presence of genets of varying ages in Population 1. This suggests ongoing sexual recruitment. However, since the ratio u/v is extremely small, vegetative propagation is much more important than sexual recruitment for this taxon, which is also supported by the significant sizes of the clones represented in Figure 3a. This supports the postulate that only a small amount of sexuality is necessary to maintain high levels of genetic diversity in a long-lived clonal species (Ellstrand and Roose, 1987).
On the other hand, no tendency in the probability of clonal identity could be detected for C. edulis. A possible explanation for the homogeneity within the distribution of clones is a mosaic growth pattern involving the fractioning and dispersal of clones by vegetative movement, which has been observed for C. edulis on the island of Porquerolles (P Vandenbrouk, pers. obs.). An alternative explanation is the agamospermic capacity of C. edulis (Suehs et al, submitted for publication, Vilà et al, 1998), which could also homogenise populations. Since the model was not designed to take into account the consequences of clone fractioning and movement, nor agamospermy, the ratio u/v for C. edulis is probably invalid, as suggested by the negative c value.
Genetic consequences on the invasion process
The high genetic diversities for C. edulis and C. acinaciformis may contribute to the continued invasion of both plant taxa, since it provides a wide range of genetic variation on which natural selection can act in new environments. Past and present hybridisation is likely to contribute to the high genetic variation found in C. acinaciformis (Ellstrand and Schierenbeck, 2000). Among the consequences of such introgressive hybridisation between invasive taxa is the propagation of advantageous genotypes/evolutionary novelties (Ellstrand and Schierenbeck, 2000). This possibility is especially worrying in light of the fact that C. edulis, rather than C. acinaciformis, has proven to be a very successful and aggressive invader in the Mediterranean Basin (Lambinon, 1995; Médail, 1998; Suehs et al, 2001). Within the introgressive events occurring on Bagaud island, the possibility exists that advantageous genes are being passed from a taxon that is problematic on a large scale (C. edulis) to one which is traditionally not considered invasive due to its rarity, but exhibits aggressive clonality (C. acinaciformis). In fact, C. acinaciformis maximises seed production when crossed with C. edulis, thus reinforcing introgression of C. edulis genes to the C. acinaciformis genome (Suehs et al, in press). This could thus create new genotypic combinations with a high invasion potential. The introgression of parental genomes towards hybrids is a phenomenon that has been recognised more and more frequently in recent years (Rieseberg, 1997), with the suggestion that the hybrids resulting from backcrosses can contribute to adaptive evolution within populations, (Rieseberg and Carney, 1998), and thus to increased invasion (Ellstrand and Schierenbeck, 2000). A similar process has already been exemplified in California where hybrids contribute to the successful invasion of Carpobrotus due to the formation of additional spreading genotypes (Vilà and D'Antonio, 1998b; Weber et al, 1998). Hybridisation may contribute to the successful invasion of C. edulis and C. acinaciformis in the Mediterranean Basin.
Furthermore, the probability of clonal identity demonstrated in this study indicates that vegetative propagation is an important reproductive alternative for C. acinaciformis. This vegetative propagation is aggressive and intense, significantly faster than that of C. edulis (Suehs, unpublished data), and most likely associated with hybrid vigour. Currently, this population is expanding towards the interior of the island (Figure 1) across the Pistacia lentiscus, Rosmarinus officinalis, Erica arborea, and even Quercus ilex bushes characteristic of the surrounding thermophilic matorral (Suehs et al, 2001). In contrast, the present distribution of C. edulis in Population 2 remains constrained to the littoral belt (Figure 1). The capacity for dense, clonal growth has been noted as a characteristic contributing to the successful invasion by other plant species (Daehler, 1998; Thompson, 1991), and could significantly contribute to further invasion by C. acinaciformis.
Arnold (1997, p 173) mentioned that ‘another potential consequence of hybridisation is the replacement of one species by recombinant genotypes’. This possibility along with the above-mentioned factors associated with genetic diversity, novelty, and hybrid vigour, stress the importance of the hybridisation process occurring between Carpobrotus taxa in the Mediterranean Basin. Further studies comparing the genomic characteristics and the relative fitnesses of parental and hybrid progenies will help clarify the future consequences of hybridisation on the progress of this severe invasion.
References
Abbott RJ 1992. Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol Evol 7: 401–405.
Akeroyd JR, Preston CD (1990). Notes on some Aizoaceae naturalized in Europe. Bot J Linn Soc 103: 197–200.
Akeroyd JR, Preston CD (1993). Carpobrotus N.E. Br. In: Tutin TG, Burges NA, Chater AO, Edmonson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea Volume 1: Psilotaceae to Platanaceae. Cambridge University Press: Cambridge. pp 135.
Albert ME, D'antonio CM, Schierenbeck KA (1997). Hybridization and introgression in Carpobrotus spp. (Aizoaceae) in California. I. Morphological Evidence. Am J Bot 84: 896–904.
Albert A, Jahandiez E (1908). Catalogue des plantes qui croissent naturellement dans le département du Var, Mus. Hist. Nat. Toulon reprints, 1985, Toulon.
Arnold ML (1992). Natural hybridization as an evolutionary process. Annu Rev Ecol Syst 23: 237–261.
Arnold ML (1997). Natural Hybridization and Evolution. Oxford University Press: New York.
Barton NH, Hewitt GM (1985). Analysis of hybrid zones. Annu Rev Ecol Syst 16: 113–148.
Barton NH, Hewitt GM (1989). Adaptation, speciation and hybrid zones. Nature 341: 497–502.
Belkhir K (1999). GENETIX, Release 4.0.. Laboratoire Génome et Populations, Montpellier.
Blake ST (1969). A revision of Carpobrotus and Sarcozona in Australia, genera allied to Mesembryanthemum (Aizoaceae). Contr Queensland Herb 7: 1–65.
Carney SE, Gardner KA, Rieseberg LH (2000). Evolutionary changes over the fifty-year history of a hybrid population of sunflowers (Helianthus). Evolution 54: 462–474.
Cook RE (1983). Clonal plant populations. Am Sci 71: 244–252.
Daehler CC (1998). Variation in self-fertility and the reproductive advantage of self-fertility for an invading plant (Spartina alterniflora). Evol Ecol 12: 553–568.
D'antonio CM, Dudley TL (1995). Biological invasions as agents of change on islands versus mainlands. In: Vitousek PM, Loope, LL, Adsersen H (eds) Islands. Biological Diversity and Ecosystem Function. Springer: Berlin. Vol. 115, pp 103–121.
De Vienne D (ed) (1998). Les marqueurs moléculaires en génétique et biotechnologies végétales, 2nd edn. Institut National de la Recherche Agronomique, Paris.
Ellstrand NC, Roose ML (1987). Patterns of genotypic diversity in clonal plant species. Am J Bot 74: 123–131.
Ellstrand NC, Schierenbeck KA (2000). Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97: 7043–7050.
Ewel JJ, O'Dowd DJ, Bergelson J, Daehler CC, D'Antonio CM, Gomez LD, Gordon DR, Hobbs RJ, Holt A, Hopper KR, Hughes CE, Lahart M, Leakey RRB, Lee WG, Loope LL, Lorence DH, Louda SM, Lugo AE, McEvoy PB, Richardson DM, Vitousek PM (1999). Deliberate introductions of species: research needs. BioScience 49: 619–630.
Fernandes RB (1972). Mesembryanthemaceae. Ann Soc Broteriana 38: 127–136.
Gallagher KG, Schierenbeck KA, D'Antonio CM (1997). Hybridization and introgression in Carpobrotus spp. (Aizoaceae) in California II. Allozyme evidence. Am J Bot 84: 905–911.
Gouffe De La Cour M (1813). Mémoire sur les végétaux exotiques qui peuvent être naturalisés dans les départements méridionaux de la France, suivi de la liste des plantes rares qui ont fleuri et de celles qui ont fructifié dans divers jardins de Marseille principalement dans le jardin de botanique et de naturalisation de cette ville. Mém Acad Marseille 11: 149–259.
Grant V (1971). Plant Speciation. Columbia University Press: New York, NY.
Hamrick JL, Linhart YB, Mitton JB (1979). Relationship between life history characteristics and electrophoretically detectable genetic variation in plants. Annu Rev Ecol Syst 10: 173–200.
Harada Y, Kawano S, Iwasa Y (1997). Probability of clonal identity: inferring the relative succes of sexual versus clonal reproduction from spatial genetic patterns. J Ecol 85: 591–600.
Haslam SM, Sell PD, Wolseley PA (1977). A Flora of the Maltese Islands. Malta University Press: Msida, Malta.
Higgins SI, Richardson DM, Cowling RM (1996). Modeling invasive plant spread: the role of plant environment interactions and model structure. Ecology 77: 2043–2054.
Huxley A, Taylor W (1977). Flowers of Greece and Aegean. Chatto & Windus: London.
Lambinon J (1995). Carpobrotus edulis (L.) N. E. Br and C. acinaciformis (L.) L. Bolus. In: Jeanmonod D, Burdet HM (eds) Notes et contributions à la flore Corse XI, Candollea 50: 564–565.
Macdonald IAW, Graber DM, Debenedetti S, Groves RH, Fuentes ER (1988). Introduced species in nature reserves in Mediterranean-type climatic regions of the world. Biol Conserv 44: 37–66.
Maki M, Murata J (2001). Allozyme analysis of the hybrid origin of Arisaema ehimense (Araceae). Heredity 86: 87–93.
Mathsoft (1999). S-PLUS 2000 Professional Edition for Windows, Release 2. MathSoft, Inc.: Seattle, WA.
Médail F (1998). Flore et végétation des îles satellites (Bagaud, Gabinière, Rascas) du parc national de Port-Cros (Var S. E. France). Sci Rep Port-Cros Natl Park 17: 55–80.
Médail F (1999). Ecologie, biologie et structure génétique des griffes-de-sorcière (Carpobrotus ssp.) végétaux exotiques envahissants dans le Parc National de Port-Cros, Institut Méditerranéen d'Ecologie et de Paléoécologie (IMEP, CNRS), case 461, 13397 Marseille cedex 20, France. Marseille.
Nei M (1973). Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70: 3321–3323.
Nei M (1977). F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet 41: 225.
Nei M (1987). Molecular Evolutionary Genetics. Columbia University Press: New York, NY.
Raymond M, Rousset F (1995). GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Heredity 86: 248–249.
Rieseberg LH (1997). Hybrid origins of plant species. Annu Rev Ecol Syst 28: 359–389.
Rieseberg LH (2000). Crossing relationships among ancient and experimental sunflower hybrid lineages. Evolution 54: 859–865.
Rieseberg LH, Carney SE (1998). Tansley Review No. 102. Plant hybridization. New Phytol 140: 599–624.
Sokal RR, Rohlf FJ (1995). Biometry, 3rd edn. Freeman and Company: New York.
Statsoft France (1997). STATISTICA pour Windows (manuel du programme), Release 5.1. StatSoft France: Paris.
Suehs CM, Affre L, Médail F . The invasion dynamics of two introduced succulents, Carpobrotus affine acinaciformis and C. edulis (Aizoaceae), on a Mediterranean Island: II. Reproductive alternatives. Heredity, in press.
Suehs CM, Médail F, Affre L (2001). Ecological and genetic features of the invasion by the alien Carpobrotus plants in Mediterranean island habitats. In: Brundu G, Brock J, Camarda I, Child L, Wade M (eds) Plant Invasions: Species Ecology and Ecosystem Management. Backhuys Publishers: Leiden. pp 145–158.
Thompson JD (1991). The biology of an invasive plant. What makes Spartina anglica so successful? BioScience 41: 393–400.
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (eds) (1964). Flora Europaea. Cambridge University Press: Cambridge. Vol. 1.
Vilà M, D'Antonio CM (1998a). Fruit choice and seed dispersal of invasive vs. noninvasive Carpobrotus (Aizoaceae) in coastal California. Ecology 79: 1053–1060.
Vilà M, D'Antonio CM (1998b). Hybrid vigor for clonal growth in Carpobrotus (Aizoaceae) in coastal California. Ecol Appl 8: 1196–1205.
Vilà M, Weber E, D'Antonio CM (1998). Flowering and mating system in hybridizing Carpobrotus (Aizoaceae) in coastal California. Can J Bot 76: 1165–1169.
Vilà M, Weber E, D'Antonio CM (2000). Conservation implications of invasion by plant hybridization. Biol Inv 2: 207–217.
Vitousek PM, D'Antonio CM, Loope LL, Westbrooks R (1996). Biological invasions as global environmental change. Am Sci 84: 468–478.
Weber EF, Vilà M, Albert M, D'Antonio CM (1998). Invasion by hybridization: Carpobrotus in coastal California. In: Starfinger U, Edwards K, Kowarik I, Williamson M (eds) Plant Invasions: Ecological Mechanisms and Human Responses. Backhuys Publishers: Leiden. pp 275–281.
Weir BS, Cockerham CC (1984). Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370.
Wendel JF, Weeden NF (1990). Visualization and interpretation of plant isozymes. In: Soltis DE, Soltis PS (eds) Isozymes in Plant Biology. Chapman & Hall: London. pp 5–45.
Williamson M (1996). Biological Invasions. Chapman & Hall: London.
Wisura W, Glen HF (1993). The South African species of Carpobrotus (Mesembryanthema–Aizoaceae). Contr Bolus Herb 15: 76–107.
Wright W (1965). The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19: 395–420.
Acknowledgements
We thank Patrice D'Onofrio, Patrick Vandenbrouk, France di Giusto, Natalia Diaz-Burlinson, and Eric Vidal for their help in the field and laboratory. John Thompson as well as two anonymous reviewers are also especially appreciated for their helpful comments. This work was supported by the National Park of Port-Cros (Contract No. 97.029.83400), EPIDEMIE (European Vth Framework Programme EVK2-2000-00736) and INVABIO (Ministère de l'Aménagement du Territoire et de l'Environnement, Subvention No. 01113).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suehs, C., Affre, L. & Médail, F. Invasion dynamics of two alien Carpobrotus (Aizoaceae) taxa on a Mediterranean island: I. Genetic diversity and introgression. Heredity 92, 31–40 (2004). https://doi.org/10.1038/sj.hdy.6800374
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800374
Keywords
This article is cited by
-
Genetic structuring and invasion status of the perennial Ambrosia psilostachya (Asteraceae) in Europe
Scientific Reports (2023)
-
The road to invasion: fine-grained distribution and suitability model for Carpobrotus sp. pl., a plant invader on a small Mediterranean island
Folia Geobotanica (2021)
-
Combined effects of land-use intensification and plant invasion on native communities
Oecologia (2020)
-
Native arthropods on exotic sand dune flowers: consideration of sample size and number for investigating rare species and sparse communities
Arthropod-Plant Interactions (2017)
-
Anthropogenic subsidies mitigate environmental variability for insular rodents
Oecologia (2013)