Abstract
Objective:
To compare the short-term effect of a high milk and a high meat intake, identical in protein amount, on bone turnover during prepuberty.
Setting:
A University Department.
Design and Subjects:
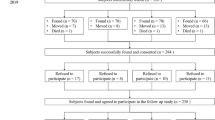
From 28, randomly recruited, 8-year-old boys, first 14 were assigned to the milk group and next 14 to the meat group. In each group, 12 boys finished the dietary intervention.
Intervention:
Milk (1.5 l/day) and meat (250 g/d), both containing ∼53 g of protein, were given together with the habitual diet for 7 days. At baseline and day−7, serum osteocalcin (s-OC), bone-specific alkaline phosphatase (s-BAP) and C-terminal telopeptides of type I collagen (s-CTX) were measured (immunoassay) and dietary intake was estimated (a 3-day weighted food record).
Results:
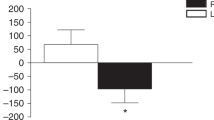
Baseline s-OC, s-BAP and s-CTX were not significantly different between the groups. After 7 days, the average protein intake increased in both groups by 47.5 g; the milk group had higher (P<0.0001) calcium intake; s-OC and s-CTX decreased (P⩽0.04) in the milk group (−30.9%; −18.7%, respectively) compared with the meat group (+6.4%; −1.0%, respectively) and s-BAP decreased (P=0.06) both in the milk (−3.9%) and the meat group (−7.5%).
Conclusions:
At the equal protein intake, milk, but not meat, decreased bone turnover in prepubertal boys after 7 days. This effect was probably due to some milk-derived compounds, rather than to the total protein intake. Future studies should elucidate the mechanism(s) of milk-related decline of bone turnover and its relevance for peak bone mass during growth.
Sponsorship:
University PhD scholarships.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Alexander J, Andressen SA, Aro A, Becker W, Lyhne N, Porsdottir I (2004). Nordic Nutrition Recommendations. Integrating Nutrition and Physical Activity. Norden: Copenhagen.
Alexy U, Remer T, Manz F, Neu CM, Schoenau E (2005). Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr 82, 1107–1114.
Andersen E, Hutchings B, Jansen J, Nyholm M (1982). Heights and weights of Danish children. Ugeskr Laeger 144, 1760–1765.
Aoe S, Toba Y, Yamamura J, Kawakami H, Yahiro M, Kumegawa M et al. (2001). Controlled trial of the effects of milk basic protein (MBP) supplementation on bone metabolism in healthy adult women. Biosci Biotechnol Biochemi 65, 913–918.
Baroncelli GI, Bertelloni S, Sodini F, Saggese G (2005). Osteoporosis in children and adolescents: etiology and management. Paediatr Drugs 7, 295–323.
Bounds W, Skinner J, Carruth BR, Ziegler P (2005). The relationship of dietary and lifestyle factors to bone mineral indexes in children. J Am Diet Assoc 105, 735–741.
Cadogan J, Eastell R, Jones N, Barker ME (1997). Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 315, 1255–1260.
Canalis E (1993). Systemic and local factors and the maintenance of bone quality. Calcif Tissue Int 53 (Suppl 1), S90–S92.
Clowes JA, Khosla S, Eastell R (2005). Potential role of pancreatic and enteric hormones in regulating bone turnover. J Bone Miner Res 20, 1497–1506.
Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EM (2000). Effect of calcium supplementation on bone mineral accretion in gambian children accustomed to a low-calcium diet. Am J Clin Nutr 71, 544–549.
Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X et al. (2004). School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr 92, 159–168.
Du XQ, Greenfield H, Fraser DR, Ge KY, Liu ZH, He W (2002). Milk consumption and bone mineral content in Chinese adolescent girls. Bone 30, 521–528.
Fares JE, Choucair M, Nabulsi M, Salamoun M, Shahine CH, Fuleihan G (2003). Effect of gender, puberty, and vitamin D status on biochemical markers of bone remodeling. Bone 33, 242–247.
Ferrari SL (2005). Osteoporosis: a complex disorder of aging with multiple genetic and environmental determinants. World Rev Nutr Diet 95, 35–51.
Ginty F (2003). Dietary protein and bone health. Proc Nutr Soc 62, 867–876.
Goulding A, Rockell JE, Black RE, Grant AM, Jones IE, Williams SM (2004). Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc 104, 250–253.
Heaney RP (1994). The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res 9, 1515–1523.
Hoppe C, Molgaard C, Juul A, Michaelsen KF (2004). High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr 58, 1211–1216.
Hoppe C, Molgaard C, Michaelsen KF (2000). Bone size and bone mass in 10-year-old Danish children: effect of current diet. Osteoporos Int 11, 1024–1030.
Johnston CC, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC et al. (1992). Calcium supplementation and increases in bone mineral density in children. N Engl J Med 327, 82–87.
Kalkwarf HJ, Khoury JC, Lanphear BP (2003). Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr 77, 257–265.
Kanbur NO, Derman O, Kinik E (2005). The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. J Bone Miner Metab 23, 76–83.
Kelly O, Cusack S, Cashman KD (2003). The effect of bovine whey protein on ectopic bone formation in young growing rats. Br J Nutr 90, 557–564.
Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ et al. (2005). Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr 81, 175–188.
Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP (1998). Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo–controlled trial. Ann Intern Med 128, 801–809.
Seydewitz HH, Henschen M, Kuhnel W, Brandis M (2001). Pediatric reference ranges for osteocalcin measured by the Immulite analyzer. Clin Chem Lab Med 39, 980–982.
Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC (1997). Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res 12, 676–682.
Takada Y, Aoe S, Kumegawa M (1996). Whey protein stimulated the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun 223, 445–449.
Takada Y, Kobayashi N, Matsuyama H, Kato K, Yamamura J, Yahiro M et al. (1997). Whey protein suppresses the osteoclast-mediated bone resorption and osteoclast cell formation. Int Dairy J 7, 821–825.
Zhu K, Du X, Cowell CT, Greenfield H, Blades B, Dobbins TA et al. (2005). Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10–12 y in Beijing. Am J Clin Nutr 81, 1168–1175.
Acknowledgements
We thank all the children for participating in the project; bio-technicians: VJ Anker, SA Jensen and HL Petersen for their assistance with the analyses of serum samples; and T Mark for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: AZ Budek.
Contributors: AZB performed biochemical measurements of markers for bone turnover, conducted the statistical analyses and prepared the manuscript in collaboration with CH, KFM and CM. No author had a financial or personal conflict of interest related to this project.
Rights and permissions
About this article
Cite this article
Budek, A., Hoppe, C., Michaelsen, K. et al. High intake of milk, but not meat, decreases bone turnover in prepubertal boys after 7 days. Eur J Clin Nutr 61, 957–962 (2007). https://doi.org/10.1038/sj.ejcn.1602605
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602605
Keywords
This article is cited by
-
The association of dietary acid load (DAL) with estimated skeletal muscle mass and bone mineral content: a cross-sectional study
BMC Nutrition (2023)
-
The effect of milk consumption on bone and fracture incidence, an update
Aging Clinical and Experimental Research (2019)
-
Dietary protein intake and bone mineral content in adolescents—The Copenhagen Cohort Study
Osteoporosis International (2007)