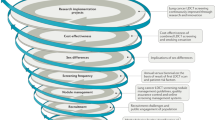
Abstract
To take lung cancer screening into national programmes, we first have to answer the question whether low-dose computed tomography (LDCT) screening and treatment of early lesions will decrease lung cancer mortality compared with a control group, to accurately estimate the balance of benefits and harms, and to determine the cost-effectiveness of the intervention.
Similar content being viewed by others
Main
Lung cancer kills more people worldwide than other malignancy. The number of deaths in the western world has fallen in the past years and this is likely to be due to a greater public awareness as well as successes in smoking cessation programmes. Unfortunately, the tobacco epidemic is still growing in Southeast Asia and China as the tobacco industry has now concentrated its sales in these regions. However, there is now a large ex-smoking population in the United States and Europe, who remain at a very high risk of developing lung cancer, which is dependent on their smoking duration before tobacco cessation. This group of individuals now exceeds current smokers in both the United States and Europe and will continue to do so over the next two to three decades. National health-care programmes would have a responsibility, if there were a proven screening tool, to provide a mechanism by which these high-risk individuals are identified and targeted for lung cancer screening. Screening must be instigated before patients develop any symptoms, as surgical resection at an early stage of the disease remains the only realistic option for a cure.
Chest X-ray and sputum cytology lung cancer screening
The earliest lung screening trial was undertaken in London with over 55 000 individuals randomised to chest X-ray every 6 months for 3 years or chest X-ray at the beginning and end of the 3-year period (Brett, 1969). No mortality difference was found between the two groups. Three major trials in the United States and one in Czechoslovakia were developed in the 1970s, as outlined in Table 1. The results of these large trials were disappointing as none of these studies showed any reduction in lung cancer mortality utilising chest X-ray, with or without, sputum cytology. However, some design features of these trials have been criticised on the basis of active early detection measures in the control arm in many of the studies, possible suboptimal selection of study populations, and of arguably inadequate sample sizes (Prorok et al, 2000). Many of these criticisms have now been taken on board by the current lung cancer screening trials.
One current trial, which has ‘usual care’ only in the control arm, is the lung component of the NCI PLCO (Prostate, Lung Colorectal and Ovarian) screening trial. In this trial, smokers are offered annual chest X-ray for 3 years, and non-smokers two annual repeat screens; the results of this study are not expected until 2010.
Low-dose computed tomography lung cancer screening: observational studies
Low-dose computed tomography (LDCT) offers a major advance in imaging technology, which was first introduced in the 1990s (Naidich et al, 1990) and later by Reeves and Kostis (2000). This is more sensitive than chest X-ray and has enabled the detection of lung tumours less than 1 cm; thus, allowing a complete scan on the thorax in less than 30 s. Randomised trials of this technology as a screening tool have not as yet been completed, but there have been a number of demonstration projects (Table 2). Early studies of note include the Early Lung Cancer Action Project (ELCAP) (Henschke et al, 1999) in 1000 high-risk smokers; the Mayo Clinic Project with 1520 individuals aged 50 years having annual sputum cytology and spiral CT screening (Swensen et al, 2000); and a 3-year mass screening programme using a mobile CT unit in Japan (Sone et al, 1998).
The ELCAP study enroled 1000 symptom-free individuals aged 60 years or more with >10 pack-years history of smoking, who were fit to undergo surgery into a study. All individuals underwent an annual spiral CT and chest X-ray. The lung cancer detection rate was 2.7% in the first year and 0.7% in the second year (incidence), and this study also demonstrated that the sensitivity of low-dose spiral CT for early lung cancer was far greater than for chest X-rays. The majority of ‘screen-detected’ tumours were at an early stage and suitable for surgery. This seminal paper by Henschke and co-workers (Henschke et al, 1999) re-ignited interest and debate in developing new lung cancer screening trials in the United States and Europe. Other demonstration projects found similar results (Table 2). The Early Lung Cancer Action Project has since been expanded to a major international collaboration, I-ELCAP, with more than 30 000 screenees (see below), with similar findings to the original New York project (Henschke et al, 2006). The authors also estimated a very high case survival rate for stage I tumours undergoing surgery. There is, however, considerable debate around the interpretation of increased survival in LDCT-diagnosed cancers, as longer survival does not necessarily equate to reduced mortality (Twombly, 2007). In addition to concerns about self-selection for surgery (or for no surgery) among stage 1 patients, the major reservation relates to overdiagnosis of tumours, which would not have been life threatening and would never have come to clinical attention in the absence of screening. The previous generation of chest X-ray trials suggested a measure of overdiagnosis (Kubik et al, 2000; Marcus et al, 2000). The much greater sensitivity of LDCT has, in turn, led to fears of an increased risk of overdiagnosis. The most balanced arguments to date concerning the IELCAP findings have been in a recent BMJ editorial (McMahon and Christiani, 2007). The authors' view is that the objective of lung cancer screening is to reduce lung cancer mortality, and it is not possible to confidently conclude this from the IELCAP study.
The one other large observational analysis is by Bach et al (2007) whose conclusions were diametrically opposed to those from by the IELCAP Consortium. Bach and colleagues used data from 3246 current or former smokers who entered into screening studies in the United States and in Italy, with follow-up for a median of 3.9 years. They used a model of predicted risk of lung cancer mortality to estimate the expected numbers of lung cancer deaths and compared these with the corresponding observed deaths; they found no decrease in the number of diagnoses of advanced lung cancers or deaths from lung cancer (38 deaths due to lung cancer observed and 38.8 expected; RR 1.0; 95% CI: 0.7–1.3; P=0.90). The authors concluded that there was no evidence of a mortality advantage with LDCT screening from this study. However, their exclusion of deaths from tumours diagnosed early in the period of observation has been criticised, as have been various other assumptions and procedures in their approach.
LDCT lung cancer screening: randomised trials
The EU-US spiral CT Collaboration was initiated in 2001 in Liverpool. Subsequent meetings throughout Europe resulted in the development of collaborative protocols on radiology, pathology, minimum datasets, treatment, as well as core LDCT protocol. This provided a mechanism by which the different trial groups could work together with the ultimate aim to pool their data, thereby enhancing the overall power of these studies and potentially reporting earlier; the concept of which was formulated in the ‘Liverpool Statement 2005’ (Field et al, 2006).
The randomised trials of LDCT are summarised in Table 3. The first major RCT lung cancer screening trial utilising LDCT was the National Lung cancer Screening Trial (NLST), which is a combination of two trials, one set up by the US National Cancer Institute (NCI) and the other by the American College of Radiology Imaging Network (ACRIN). The NLST started in 2002 and completed enroling in 2004. This study has over 50 000 former and current smokers randomised to annual LDCT or annual chest X-ray for 3 years. The major objective of this was to determine whether LDCT reduces lung cancer mortality compared with a chest X-ray arm. (http://www.cancer.gov/NLST). This trial will be completed in 2009 and aims to report in 2012; it is designed to have a 90% power to detect a mortality reduction of 20%.
The NELSON trial was launched in 2003 in the Netherlands and Belgium (van Iersel et al, 2007), and now incorporates centres in Denmark. This trial is designed to compare lung cancer mortality in a group randomised to LDCT screening with a control group, without screening. This trial aims to report in 2014 and with 20 000 recruits and is designed to have a power of 80%, significance level of 0.05 to detect a mortality reduction of 20%; a 95% compliance in the screen group, a 5% contamination rate in the control group and 10 years follow-up after randomisation. A great deal of attention was focused on the selection of patients for NELSON in order to focus on the highest risk groups and thus reduce the cost of the RCT but retain the power of the study. Potential study participants were approached by letter with a questionnaire on their smoking exposure and whether they wished to be included in the trial. The questionnaire was initially sent to 335 441 men and women aged 50–75 years old. On the basis of this data set the selection criteria were developed, depending on the duration of smoking, duration of smoking cessation in ex-smokers, number of cigarettes smoked per day, and the mean estimated expected lung cancer mortality rate. In this trial, LDCT screening takes place in years 1, 2, and 4, with 10 years of follow-up. The trial has 20 000 individuals randomised in equal numbers to LDCT or ‘usual care’.
A number of small trials have been initiated in anticipation of combination with partner studies or a future meta-analysis. These include the ItaLung and Dante Trials in Italy (Picozzi et al, 2005; Infante et al, 2007).
The French randomised pilot study, Depiscan, comparing LDCT and chest X-ray recently reported its baseline findings (Blanchon et al, 2007); in this the selection of participants was undertaken by General Practioners (GPs) and occupational physicians. Eligible subjects were males and females aged 50–75 years with either a current or former smoking history of at least 15 cigarettes per day for 20 years. The screening was undertaken annually for 2 years. The objective was to enrol 1000 subjects; 765 have been recruited with 621of these having complete imaging baseline data. Non-compliance was an important issue in this study and the recruitment took twice as long as envisaged. Eight lung cancers were detected in the LDCT arm (2.4%) and one (<1%) in the chest X-ray arm.
National lung cancer screening programme
To date, we do not have the results of any randomised trials, which can provide adequate evidence to justify the instigation of a National Lung Cancer Screening Programme. The results of the NLST and NELSON studies are eagerly awaited. The unanswered question that remains in the United Kingdom is whether either of these studies will provide adequate information on their own to justify the implementation of a UK National Screening Programme? Although the combined US study is large and should have precise results, the use of an active screening regime in the control group may raise problems of interpretation. The NELSON study has adequate power for a substantial benefit in a high-risk group, but a lower baseline lung cancer mortality or smaller benefit than anticipated may jeopardise a conclusive result.
The UK National Screening Committee has determined 22 criteria for the viability, effectiveness, and appropriateness of a screening programme (http://www.nsc.nhs.uk/uk_nsc/uk_nsc_ind.htm); 20 of which are relevant to LDCT lung cancer screening. Black et al (2007) have undertaken a systematic review of the literature to ascertain whether there was evidence for any clinical effectiveness utilising LDCT for lung cancer screening. This extremely detailed review was undertaken at the time when there was a paucity of real data, and thus their conclusions were drawn from two small trials with very variable results. Not surprisingly, their conclusion stated that there was insufficient evidence at the time to support LDCT screening.
The current lack of evidence and the possibility of inconclusive results from relatively small group of current trials would suggest that a UK trial would make a valuable contribution to the research effort worldwide and answer questions particularly pertinent to the UK health environment. It is a salutary fact that four decades after the development of this ‘technology’, we still do not have experimental evidence for or against the implementation of this screening modality. Lung cancer kills more individuals in the United Kingdom than any other malignancy. Our responsibility is not only to determine whether LDCT screening and treatment of early lesions will decrease lung cancer mortality compared with a control group without screening but also to test this against the criteria outlined by the UK Screening Committee, especially those concerning cost-effectiveness. A useful aid to cost-effectiveness is the ability to select a population at sufficiently high risk to give a substantial harvest of tumours in return for the screening activity. The Liverpool Lung Project Risk Model provides an opportunity for this (Field et al, 2007; Cassidy et al, 2008). The risk groups selected are those for whom the benefits of the screening will outweigh the likely harms.
The cost-effectiveness of lung cancer LDCT screening has been estimated by a number of groups, which were reviewed by Black et al (2006), who found the current estimates difficult to interpret and certainly not definitive. In response to a request from the UK National Cancer Research Institute, Whynes (2008) developed a simple and transparent economic model based on UK costings and the empirical clinical data are currently available. The UK cost-effectiveness model used a simple, deterministic approach to the modelling of a screening regimen. The model required only a limited number of parameters. The expected mortality gain as a result of screening was estimated by combining published survival data from screened and unscreened cohorts with routinely published national mortality figures. Conservative costs were estimated where there was uncertainty over any specific parameter, thus probably resulting in less cost-effective screening. The incremental cost-effectiveness ratio of a single CT screen among a high-risk male population was calculated to be around £14 000 per quality-adjusted life year gained, if the anticipated mortality benefit was indeed observed. Sensitivity analysis was carried out with a range of differing assumptions, providing a range of cost-effectiveness ratios as high as £21 000 or as low as around £6000. In the United Kingdom, the National Institute and Clinical Excellence (NICE) evaluated both clinical and cost-effectiveness when deciding on recommendations to implement new interventions. Currently, NICE considered ICERs below £20 000 per QUALY as definitely acceptable and costs up to £30 000 as suitable for consideration.
The approval of any future lung cancer screening trial will evidently be dependent on costings in line with current political health economics; however, this defining factor was not applicable for either breast cancer screening, which was set up after the Forest Report in 1985 (Gerard et al, 1997), or cervical cancer screening, which was set up in 1992 (Quinn et al, 1999). The most efficient way of controlling cost, however, will be to screen those individuals who are at high risk of developing the disease. There has been increasing interest in developing methods for individual risk prediction for lung cancer. Models have been developed for use within high-risk groups (Bach et al, 2003), and for the general population (van Klaveren et al, 2002), which rely only on age and smoking. Epidemiological risk factors usually show poor discrimination between those ‘who do’ and ‘do not’ develop disease (Wald et al, 1999), but lung cancer is an exception, in that a high proportion of cases are attributable to one risk factor, smoking. The predictive accuracy of lung cancer risk models may be further improved by the addition of other epidemiological risk factors, including smoking history variables, environmental tobacco smoke, family history of cancer, prior respiratory disease, and occupational exposures (dust and asbestos) (Cassidy et al, 2007, 2008; Spitz et al, 2007). The Liverpool Lung Project (LLP) (Field et al, 2005) has recently developed a method to calculate absolute risk of lung cancer over a defined period, based on data from a case–control study of lung cancer in Liverpool. Significant risk factors in the final model were smoking duration, family history of lung cancer, history of non-pulmonary malignant tumour, history of pneumonia, and occupational exposure to asbestos. These factors were combined with published age- and sex-specific incidence rates to give absolute probability of lung cancer development within 5 years. In comparison with previous lung cancer prediction models, the LLP risk model has distinctive strengths. First, the predictor variables are all explicitly defined and can be readily assessed at the time of patient presentation, and secondly, patients can be assigned to their appropriate risk class on the basis of information from the initial history alone. The LLP Risk Model requires rigorous validation in a separate population.
Conclusion
Currently, the treatment of advanced lung cancer is inadequate and, thus, there is an urgent pressure to implement screening programmes in many countries. In the United Kingdom, no decision will be made until we have the results of the current international RCT trials and, hopefully, those from a future UK lung cancer screening RCT. However, time is not on our side with over 32 000 individuals a year dying from lung cancer in the United Kingdom, and this statistic alone should accelerate progress in reaching a conclusion concerning the feasibility of lung cancer screening.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB (2007) Computed tomography screening and lung cancer outcomes. JAMA 297: 953–961
Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, Hsieh LJ, Begg CB (2003) Variations in lung cancer risk among smokers. J Natl Cancer Inst 95: 470–478
Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, Ayres J, Bain L, Thomas S, Godden D, Waugh N (2006) The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews. Health Technol Assess 10 (3): iii–iv, ix–x, 1–90
Black C, de Verteuil R, Walker S, Ayres J, Boland A, Bagust A, Waugh N (2007) Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax 62: 131–138
Blanchon T, Brechot JM, Grenier PA, Ferretti GR, Lemarie E, Milleron B, Chague D, Laurent F, Martinet Y, Beigelman-Aubry C, Blanchon F, Revel MP, Friard S, Remy-Jardin M, Vasile M, Santelmo N, Lecalier A, Lefebure P, Moro-Sibilot D, Breton JL, Carette MF, Brambilla C, Fournel F, Kieffer A, Frija G, Flahault A (2007) Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 58: 50–58
Brett GZ (1969) Earlier diagnosis and survival in lung cancer. BMJ 4: 260–262
Cassidy A, Duffy SW, Myles JP, Liloglou T, Field JK (2007) Lung cancer risk prediction: a tool for early detection. Int J Cancer 120: 1–6
Cassidy A, Myles J, van-Tongeren M, Page R, Liloglou T, Duffy S, Field J (2008) The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 98: 270–276
Chong S, Lee KS, Chung MJ, Kim TS, Kim H, Kwon OJ, Choi YH, Rhee CH (2005) Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 20: 402–408
Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, Heindel W (2002) Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 222: 773–781
Field JK, Cassidy A, Myles J, Liloglou T, Xinarianos G, Smith D, Asaf N, Page R, Duffy S (2007) Biomarkers and risk models for stratification of high risk individuals. J Thora Oncol 2: 176
Field JK, Smith DL, Duffy S, Cassidy A (2005) The Liverpool Lung Project research protocol. Int J Oncol 27: 1633–1645
Field JK, Smith RA, Duffy SW, Berg CD, van Klaveren R, Henschke CI, Carbone D, Postmus PE, Paci E, Hirsch FR, Mulshine JL (2006) The Liverpool Statement 2005: priorities for the European Union/United States spiral computed tomography collaborative group. J Thorac Oncol 1: 497–498
Ford LG, Minasian LM, McCaskill-Stevens W, Pisano ED, Sullivan D, Smith RA (2003) Prevention and early detection clinical trials: opportunities for primary care providers and their patients. CA Cancer J Clin 53: 82–101
Frost JK, Ball Jr WC, Levin ML, Tockman MS, Baker RR, Carter D, Eggleston JC, Erozan YS, Gupta PK, Khouri NF, March BR, Stitik FP (1984) Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis 130: 549–554
Gerard K, Brown J, Johnston K (1997) UK breast screening programme: how does it reflect the Forrest recommendations? J Med Screen 4: 10–15
Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, Ascher S, Bailey W, Brewer B, Church T, Engelhard D, Ford M, Fouad M, Freedman M, Gelmann E, Gierada D, Hocking W, Inampudi S, Irons B, Johnson CC, Jones A, Kucera G, Kvale P, Lappe K, Manor W, Moore A, Nath H, Neff S, Oken M, Plunkett M, Price H, Reding D, Riley T, Schwartz M, Spizarny D, Yoffie R, Zylak C (2005) Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 47: 9–15
Gohagan JK, Prorok PC, Hayes RB, Kramer BS (2000) The prostate, lung, colorectal and ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 21: 251S–272S
Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP (1999) Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354: 99–105
Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS (2006) Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 355: 1763–1771
Henschke CI (2000) Early lung cancer action project: overall design and findings from baseline screening. Cancer 89: 2474–2482
Infante M, Lutman FR, Cavuto S, Brambilla G, Chiesa G, Passera E, Angeli E, Chiarenza M, Aranzulla G, Cariboni U, Alloisio M, Incarbone M, Testori A, Destro A, Cappuzzo F, Roncalli M, Santoro A, Ravasi G (2007) Lung cancer screening with spiral CT baseline results of the randomized DANTE trial. Lung Cancer
Kubik AK, Parkin DM, Zatloukal P (2000) Czech Study on Lung Cancer Screening: post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 89: 2363–2368
MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW (2004) Screening for lung cancer using low dose CT scanning. Thorax 59: 237–241
Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, Prorok PC (2000) Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst 92: 1308–1316
McMahon PM, Christiani DC (2007) Computed tomography screening for lung cancer. BMJ 334: 271
Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N (1984) Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest 86: 44–53
Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI (1990) Low-dose CT of the lungs: preliminary observations. Radiology 175: 729–731
Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H (2002) Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 122: 15–20
Pedersen JH, Dirksen A, Olsen JH (2002) Screening for lung cancer with low-dosage CT. Ugeskr Laeger 164: 167–170
Picozzi G, Paci E, Lopez Pegna A, Bartolucci M, Roselli G, De Francisci A, Gabrielli S, Masi A, Villari N, Mascalchi M (2005) Screening of lung cancer with low dose spiral CT: results of a three year pilot study and design of the randomised controlled trial ‘Italung-CT’. Radiol Med (Torino) 109: 17–26
Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, Mandel JS, Oberman A, O'Brien B, Oken MM, Rafla S, Reding D, Rutt W, Weissfeld JL, Yokochi L, Gohagan JK (2000) Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials 21: 273S–309S
Quinn M, Babb P, Jones J, Allen E (1999) Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ 318: 904–908
Reeves AP, Kostis WJ (2000) Computer-aided diagnosis for lung cancer. Radiol Clin North Am 38: 497–509
Rossi A, Maione P, Colantuoni G, Gaizo FD, Guerriero C, Nicolella D, Ferrara C, Gridelli C (2005) Screening for lung cancer: new horizons? Crit Rev Oncol Hematol 56: 311–320
Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, Kakinuma R, Ohmatsu H, Nagai K, Nishiyama H, Matsui E, Eguchi K (2002) Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 20: 911–920
Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, Asakura K (1998) Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 351: 1242–1245
Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, Shete S, Etzel CJ (2007) A risk model for prediction of lung cancer. J Natl Cancer Inst 99: 715–726
Stephenson SM, Mech KF, Sardi A (2005) Lung cancer screening with low-dose spiral computed tomography. Am Surg 71: 1015–1017
Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, Aughenbaugh GL, Zink FE, Hillman SL, Noetzel GR, Marks RS, Clayton AC, Pairolero PC (2002) Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 165: 508–513
Swensen SJ, Viggiano RW, Midthun DE, Muller NL, Sherrick A, Yamashita K, Naidich DP, Patz EF, Hartman TE, Muhm JR, Weaver AL (2000) Lung nodule enhancement at CT: multicenter study. Radiology 214: 73–80
Tiitola M, Kivisaari L, Huuskonen MS, Mattson K, Koskinen H, Lehtola H, Zitting A, Vehmas T (2002) Computed tomography screening for lung cancer in asbestos-exposed workers. Lung Cancer 35: 17–22
Twombly R (2007) Lung cancer screening debate continues despite international CT study results. J Natl Cancer Inst 99: 190–195
van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, Prokop M, Habbema JD, Oudkerk M, van Klaveren RJ (2007) Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 120: 868–874
van Klaveren RJ, de Koning HJ, Mulshine J, Hirsch FR (2002) Lung cancer screening by spiral CT. What is the optimal target population for screening trials? Lung Cancer 38: 243–252
Wald NJ, Hackshaw AK, Frost CD (1999) When can a risk factor be used as a worthwhile screening test? BMJ 319: 1562–1565
Whynes DK (2008) Could CT screening for lung cancer ever be cost effective in the United Kingdom? Cost Eff Resour Alloc 6: 5
Yau G, Lock M, Rodrigues G (2007) Systematic review of baseline low-dose CT lung cancer screening. Lung Cancer 58: 161–170
Acknowledgements
This work was supported by the Roy Castle Lung Cancer Foundation and CRUK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Field, J., Duffy, S. Lung cancer screening: the way forward. Br J Cancer 99, 557–562 (2008). https://doi.org/10.1038/sj.bjc.6604509
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604509
Keywords
This article is cited by
-
STAT1 modification improves therapeutic effects of interferons on lung cancer cells
Journal of Translational Medicine (2015)
-
Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom
British Journal of Cancer (2014)
-
Evaluation of FTIR Spectroscopy as a diagnostic tool for lung cancer using sputum
BMC Cancer (2010)
-
Treatment options for small cell lung cancer – do we have more choice?
British Journal of Cancer (2010)
-
Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON)
British Journal of Cancer (2010)