Abstract
Study design:
Discussion of issues and development of consensus.
Objective:
Present the background, purpose, development process, format and definitions of the International Spinal Cord Injury Pain (ISCIP) Classification.
Methods:
An international group of spinal cord injury (SCI) and pain experts deliberated over 2 days, and then via e-mail communication developed a consensus classification of pain after SCI. The classification was reviewed by members of several professional organizations and their feedback was incorporated. The classification then underwent validation by an international group of clinicians with minimal exposure to the classification, using case study vignettes. Based upon the results of this study, further revisions were made to the ISCIP Classification.
Results:
An overall structure and terminology has been developed and partially validated as a merger of and improvement on previously published SCI pain classifications, combined with basic definitions proposed by the International Association for the Study of Pain and pain characteristics described in published empiric studies of pain. The classification is designed to be comprehensive and to include pains that are directly related to the SCI pathology as well as pains that are common after SCI but are not necessarily mechanistically related to the SCI itself.
Conclusions:
The format and definitions presented should help experienced and non-experienced clinicians as well as clinical researchers classify pain after SCI.
Similar content being viewed by others
Introduction
The purpose of the International Spinal Cord Injury Pain (ISCIP) Classification is to offer a method for classifying pain reported by persons with spinal cord injury (SCI), where pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.1
Although there is consensus in the medical community that pain after SCI is common, there has historically been no consensus on how to define and classify it. This has led to an ever-increasing number (over 29 by 2002) of different classification schemes reported in the literature.2 As classifications are built upon definitions, and prevalences of pain types are calculated based upon identified defined pain types, it should not be surprising that there are widely varying estimates of the prevalence of various types of pain after SCI. For example, the prevalence of visceral pain has been estimated to be in the range from 5 to 34%,3, 4, 5 whereas the prevalence of neuropathic pain thought to be due to spinal cord damage and experienced below the level of injury has been estimated to be anywhere from 14 to 40%.4, 5, 6, 7
Some of the variance in the reported prevalences is presumably due to methodological aspects of study design, for example, the time that has elapsed since injury at the point when a question on the presence of pain is asked, the threshold of intensity or discomfort at which pain or severe pain is defined, and questionnaire response rates or skewed population sampling. Estimates of the overall prevalence of pain after SCI range from 25 to 96%,8 whereas for severe pain, the prevalence ranges from 30 to 51%.9 However, a key problematic aspect is the lack of consistent definitions of SCI pain categories, which makes comparisons between studies difficult even if the other aspects of the design are controlled for.9 This is most evident in differentiating between subtypes of pain of somewhat similar presentation, for example, visceral pain and neuropathic abdominal pain or pain due to nerve root damage and neuropathic pain related to spinal cord damage occurring at the level of injury. Other entities that may cause confusion include neuropathic pains related to spinal cord damage with associated autonomic features, which may mimic complex regional pain syndrome or secondary effects of an initial injury, for example, root injury leading to myofascial pain in an affected myotome.
Complicating matters even further, the literature is replete with different labels presumably identifying the same entities. Neuropathic pain thought to be due to damage to the spinal cord and presenting topographically in the dermatomes adjacent to the damaged segments has been variously called end-zone pain, lesional hyperesthesia, at-level central pain, segmental pain and at-level spinal cord pain. Neuropathic pain thought to be due to damage to the spinal cord and presenting topographically in the dermatomes below the damaged segments has been variously called spinal cord pain, phantom pain, diffuse pain, central remote pain, deafferentation pain, below level central pain and SCI pain.5, 10, 11, 12, 13, 14, 15
Such a confusion of number and nature of pain subtypes, and terms used, obviously impedes communication between clinicians, researchers and others. The authors agreed that it would be worthwhile to create a consensus classification based on international input and state-of-the-art basic and clinical scholarship. This paper describes the background, purpose, development process, format and definitions of the ISCIP Classification.
Methods
An international group of clinicians and researchers was convened on 6 and 7 March 2009 in London, UK, to consider the development of a consensus SCI pain classification. Their purpose was to replace the various existing classifications with one that was based on state-of-the-art science, simple and potentially acceptable as the consensus taxonomy by all interested in treating and/or studying SCI pain.
The 15 members (all coauthors of this paper) of the ISCIP Classification group were invited by the first author because they had written about SCI pain and SCI pain taxonomies in the last decade, or were clinician representatives of the major world pain or SCI organizations, and were expected to be able to make a meaningful contribution to the development of a consensus classification. The expertise represented by the members, who hailed from six countries: Australia, Denmark, Israel, Germany, Sweden and the United States, included basic SCI pain research, clinical SCI pain research, SCI clinical care, physical therapy, psychology, SCI consumer advocacy, anesthesiology, neurology, and physical medicine and rehabilitation.
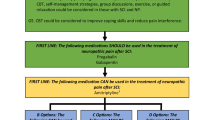
At the London meeting, the most prominent existing pain and SCI pain classification schemes were discussed, as well as the potential for integrating them into a useful consensus scheme that was simple, feasible, and in line with state-of-the-art thinking and research on the etiology of pain. Over the next year, the panel developed a draft classification based upon some of the major and more recent previously proposed classifications, using the electronic exchange of successive drafts.5, 9, 14, 16 The final draft was sent out for review by all of the major SCI and pain organizations, including: the American Spinal Injury Association (ASIA), the American Pain Society, the International Association for the Study of Pain (IASP), the International Spinal Cord Society (ISCoS), and the Academy of Spinal Cord Injury Professionals. All the organizations gave written feedback, which was reviewed by the group and incorporated as appropriate into a revised classification (see Figure 1).
The classification was then tested for utility and reliability using a process whereby 75 different clinical vignettes (brief case histories of hypothetical individuals with SCI pain), incorporated into a survey, were distributed to a random sample of members of ASIA and ISCoS. For each vignette, these clinicians were asked to classify the pain described. The correct classification with which their answers were compared was that made by a consensus of three panel members. The overall correctness in determining the pain type, when calculated using strict criteria, was 68%. For some specific subtypes, it was higher (for example, musculoskeletal pain: 84%) while for others it was lower (for example, neuropathic at-level SCI pain: 57%).17 Based upon analysis of misclassifications, several of the definitions and descriptions of pain types within the ISCIP Classification manual were revised. This was done to clarify the characterization of certain subtypes of pain and to further emphasize specific definitions which have changed over time (such as the IASP definition of neuropathic pain18) or may not be all that intuitive at first glance.
Results
Format and definitions of the ISCIP Classification
The classification organizes SCI pains hierarchically into three tiers (see Figure 1):
-
The first tier (Tier 1) includes the types of nociceptive pain, neuropathic pain, other pain, and unknown pain.
-
The second tier (Tier 2) includes for the neuropathic and nociceptive categories various subtypes of pains identified in previous SCI pain classifications.5, 9, 14, 16
-
The third tier (Tier 3) is used to specify the primary pain source at the organ level as well as the pathology, if either is known. For the other pain category, this tier is used to specify distinct recognized pain entities or syndromes which do not fulfill the criteria for nociceptive or neuropathic pain.
The instructions for classifying the pain or pains reported by an individual are as follows.
Classify each pain separately, by checking on the Figure 1 form or its equivalent only one box in each of the first two tiers, if known, and if a checkbox is provided. Next, for the third tier, specify the primary pain source at the organ level as well as the pathology, if either is known. For those pain syndromes or entities that do not fulfill the criteria for nociceptive or neuropathic pain as described below, state the specific entity or syndrome in tier 3. If a pain seems to have both nociceptive and neuropathic characteristics and is not a pain that would be considered an other pain (see below), if possible separate it into its component etiologies. If pain subtype cannot be determined, for example, it is unknown whether a neuropathic pain felt at the level of injury is due to a spinal cord or root injury or due to a thoracotomy the pain is only classified in Tier 1 (in this example as neuropathic pain).
Neuropathic pain directly attributable to SCI should be described in relation to the neurological and not the skeletal (bony) level of injury. The neurological level of injury (NLI) is defined by the International Standards for the Neurological Classification of Spinal Cord Injury as the most caudal dermatome with normal sensation for both pinprick and light touch or the myotome with normal motor function. As this may differ between the two sides of the body, the most caudal level with normal sensation or motor function is used.19 These pains may occur in persons with either complete or incomplete injuries as defined by the International Standards.
Nociceptive pain types
The proposed nomenclature of the IASP defines nociceptive pain as pain arising from activation of nociceptors, where a nociceptor is defined as a peripheral nerve ending or a sensory receptor that is capable of transducing and encoding noxious stimuli.1
Musculoskeletal (nociceptive) pain refers to pain occurring in a region where there is at least some preserved sensation, and that is, believed to be arising from nociceptors within musculoskeletal structures (muscles, tendons, ligaments, joints, bones). It may occur at any location where there are musculoskeletal structures, including areas below the NLI. The presence of Musculoskeletal pain is suggested by one or more of the following:
-
The pain is increased/decreased or otherwise changed by movement or a change in position
-
Tenderness of musculoskeletal structures on palpation
-
Evidence of skeletal pathology on imaging, that is, consistent with the pain presentation
-
Endorsement of the pain descriptors ‘dull’ or ‘aching’
-
Response of the pain to anti-inflammatory or opioid medications. Although neuropathic pain (see below) can respond to opioids, nociceptive pain is typically more responsive.
Examples include: pain resulting from joint arthritis, spinal fractures, muscle injury, rotator cuff tendinopathy and muscle spasms.5, 9, 14, 16
Failure to find evidence of musculoskeletal pathology underlying pain, that is, located at or below the NLI or failure of the pain to respond to treatment directed at such pathology may indicate the presence of at-level SCI (neuropathic) pain or below-level SCI (neuropathic) pain (see below).
Visceral (nociceptive) pain refers to pain located (usually) in the thorax, abdomen or pelvis, which is believed to be primarily generated in visceral structures. The presence of Visceral pain is suggested by one or more of the following:
-
A temporal relationship to food intake or visceral functions (for example, constipation)
-
Tenderness of visceral structures on palpation of the abdomen
-
Evidence of visceral pathology, on imaging or other testing, that is consistent with the pain presentation
-
Endorsement of one or more of the following pain descriptors: ‘cramping’, ‘dull’ or ‘tender’
-
Associated nausea and sweating
Examples include: pain resulting from constipation, urinary tract infection, ureteral calculus, bowel impaction, cholecystitis and myocardial infarction.3, 5, 9, 14, 16
If pain is localized to the thorax, abdomen or pelvis but there is no relation of this pain to any visceral function and there is no evidence of visceral pathology, at-level SCI (neuropathic) pain or below-level (neuropathic) SCI pain should be considered as the cause. For example, a pain of a constant duration appreciated in the genital or sacral region, without there being evidence of visceral or other nociceptive pathology, should be classified as either as at-level or below-level SCI (neuropathic) pain, depending on the NLI, and not as visceral pain. On the other hand, if pain in the abdominal region develops many years after the SCI, which it does in many instances14, 15 it may suggest that even though a visceral pathology cannot be demonstrated it may be related to constipation or other visceral pathology, rather than being considered a neuropathic pain.
Other (nociceptive) pain refers to nociceptive pains that do not fall into the musculoskeletal or visceral categories. These pains may be indirectly related to the SCI (for example, pain from pressure sores and autonomic dysreflexia headache) or may be unrelated to SCI (for example, migraine).9
Neuropathic pain types
IASP now defines neuropathic pain as pain caused by a lesion or disease of the somatosensory nervous system.18
At-level SCI (neuropathic) pain refers to neuropathic pain that is experienced when a lesion or disease of a nerve root or the spinal cord is presumed to be the cause of pain. It is perceived in a segmental pattern anywhere within the dermatome of the NLI and/or within the three dermatomes below this level and not in any lower dermatomes, unless the pain is thought to be caused by damage to the cauda equina, in which case it may be perceived in lower dermatomes (extending below three dermatomes below the NLI). If the pain is found in one or more dermatomes at or below the NLI, but extends to one dermatome above the NLI, it still can be classified as at-level neuropathic pain. If there is no evidence of injury to the cauda equina or caudal nerve roots, any pain that is experienced within three dermatomes of the NLI and that also is experienced below three levels below the NLI should not be described as at-level pain. A necessary condition for classifying a pain as at-level SCI pain is that a lesion or disease must affect the spinal cord or nerve roots and that the pain is believed to arise as a result of this damage. The pain may be unilateral or bilateral. Neuropathic pain that occurs in this distribution and that cannot be attributed to spinal cord or nerve root damage should be classified as other (neuropathic) pain.
At-level SCI pain may arise from pathology in two different sites, which are often difficult to tell apart (1) the spinal cord, where the insult is to the central somatosensory system; and (2) the nerve roots, where the insult is to the peripheral somatosensory system. The presence of at-level SCI pain is suggested by characteristics such as:4, 5, 9, 16, 20, 21
-
Sensory deficits within the pain distribution
-
Allodynia or hyperalgesia within the pain distribution
-
Endorsement of one or more of the following pain descriptors: ‘hot-burning’, ‘tingling’, ‘pricking’, ‘pins and needles’, ‘sharp’, ‘shooting’, ‘squeezing’, ‘painful cold’ and ‘electric shock-like’
Pain occurring in a segmental pattern as described above, which is thought to be due to syringomyelia, should be classified as at-level SCI pain or, more specifically (using tier 3 entries), at-level SCI pain due to/associated with syringomyelia. Note that the current NLI often is higher than the original NLI in patients who develop syringomyelia following a SCI.
Below-level SCI (neuropathic) pain refers to neuropathic pain that is perceived more than three dermatomes below the dermatome of the NLI; it may extend up to the dermatome representing the NLI and the three dermatomes below the NLI. A necessary condition for classifying a pain as below-level SCI pain is that a lesion or disease must affect the spinal cord and that the pain is believed to arise as a result of this damage. Neuropathic pain that occurs in this distribution and that cannot be attributed to the spinal cord damage should be classified as other (neuropathic) pain. The presence of below-level SCI pain is suggested by characteristics such as: 4, 5, 9, 16, 20, 21, 22
-
Sensory deficits within the pain distribution
-
Allodynia or hyperalgesia within the pain distribution (for persons with incomplete injury)
-
Endorsement of one or more of the following pain descriptors: ‘hot-burning’, ‘tingling’, ‘pricking’, ‘pins and needles’, ‘sharp’, ‘shooting’, ‘squeezing’, ‘painful cold’ and ‘electric shock-like’
Below-level SCI pain can occur in persons with complete injuries and in those with incomplete injuries. Neuropathic pain associated with cauda equina damage is radicular in nature, and therefore defined as at-level SCI (neuropathic) pain, regardless of distribution. If the pain is present in the region below three dermatomes below the NLI that extends to the at-level region, the pain is classified as below-level SCI (neuropathic) pain unless the person is able to distinguish a separate at-level (neuropathic) component. If two separate pains are distinguishable within the at-level region, of which only one extends below three dermatomes below the NLI, two pain types, that is, at-level (neuropathic) and below-level (neuropathic) must be classified and documented.
Other (neuropathic) pain refers to neuropathic pain that is present above, at or below the NLI but pathologically is not related to the SCI. Examples include postherpetic neuralgia, pain associated with diabetic neuropathy or a compressive mononeuropathy such as carpal tunnel syndrome, central post-stroke pain, pain from lumbar radiculopathy in someone with incomplete tetraplegia and pain related to a multiple sclerosis spinal cord lesion unrelated to the primary SCI.9, 16 Pain that occurs at or below the NLI but is clearly attributable to nerve root avulsion should also be classified as other (neuropathic) pain.
Other pain types
Other pain is defined as pain that occurs when there is no identifiable noxious stimulus nor any detectable inflammation or damage to the nervous system responsible for the pain. It is unclear what causes the pain to develop or persist. This type of pain has been also described as dysfunctional pain.23 Examples of Other pain include: Complex Regional Pain Syndrome type I, interstitial cystitis pain, irritable bowel syndrome pain and fibromyalgia.
Other pain should only be chosen for pains that are thought to be unrelated to the underlying SCI, both temporally and mechanistically. This category should not be used, for instance, to characterize pain that appears soon after SCI with neuropathic and nociceptive qualities and associated profound autonomic changes localized to the level of injury. This latter example could be coded as at-level (neuropathic) SCI pain with an additional comment that the pain is associated with autonomic features. The nociceptive component should be coded separately as nociceptive pain.
Unknown pain types
Unknown pain refers to types of pain that cannot be assigned with any degree of certainty to any of the above categories. Unknown pain refers only to pain of unknown etiology and not to pain of seemingly mixed etiology, that is, pains with both nociceptive and neuropathic qualities, nor to defined pain syndromes of unknown etiology, like fibromyalgia. For pains that seem to have both nociceptive and neuropathic qualities the two components should be classified separately using the appropriate nociceptive, neuropathic or other subtypes. Defined pain syndromes of unknown etiology (for example, fibromyalgia) should be coded as ‘Other pain and not Unknown pain’.
Miscellaneous points
Specification of the anatomic location where each pain is perceived, pain severity, temporal pattern of pain presence and pain interference, while not specifically part of the classification, is also recommended to be included in any comprehensive evaluation of pain in persons with SCI. The International SCI Pain Basic Data Set offers a standard listing of pain sites, as well as coding schemes for these other characterizing features.24
Dysesthesias, defined by the IASP as unpleasant abnormal spontaneous or evoked sensations, should only be classified if they are said to be painful by the person experiencing such sensations.25
Discussion and conclusions
The development of a consensus classification is an imperative step to developing effective treatments of pain in persons with SCI. Pain that is not classified correctly cannot be assessed and treated appropriately, and cannot be communicated between clinicians and researchers. The ISCIP Classification offers considerable detail for recording pain types, whether neuropathic, nociceptive, neither or unknown. The ISCIP Classification, targeted to both the experienced and non-experienced clinician as well as the clinical researcher, is designed to be comprehensive and to include pains that are directly related to the SCI and pains that are common after SCI but are not necessarily mechanistically related to the SCI itself. The authors look forward to comments as well as efforts to use the classification in clinical service and research.
Data Archiving
There was no data to deposit.
References
Loeser JD, Treede RD . The Kyoto protocol of IASP basic pain terminology. Pain 2008; 137: 473–477.
Hicken BL, Putzke JD, Richards JS . Classification of pain following spinal cord injury: literature review and future directions. In: Burchiel KJ, Yezierski RP, (eds). Spinal Cord Injury Pain: Assessment, Mechanisms, Management. International Association for the Study of Pain Press: Seattle, 2002; vol. 23: 25–38.
Finnerup NB, Faaborg P, Krogh K, Jensen TS . Abdominal pain in long-term spinal cord injury. Spinal Cord 2008; 46: 198–203.
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ . A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003; 103: 249–257.
Cardenas DD, Turner JA, Warms CA, Marshall HM . Classification of chronic pain associated with spinal cord injuries. Arch Phys Med Rehabil 2002; 83: 1708–1714.
Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ . Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 1999; 81: 187–197.
Woolsey RM . Chronic pain following spinal cord injury. J Am Paraplegia Soc 1986; 9: 39–41.
Dijkers M, Bryce T, Zanca J . Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev 2009; 46: 13–29.
Bryce TN, Ragnarsson KT . Epidemiology and classification of pain after spinal cord injury. Top Spinal Cord Inj Rehabil 2001; 7: 1–17.
Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Föllinger S, Wienke C et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord 1997; 35: 446–455.
Kaplan LI, Grynbaum BB, Lloyd KE, Rusk HA . Pain and spasticity in patients with spinal cord dysfunction. Results of a follow-up study. JAMA 1962; 182: 918–925.
Burke DC . Pain in paraplegia. Paraplegia 1973; 10: 297–313.
Maury M . About pain and its treatment in paraplegics. Paraplegia 1978; 15: 349–352.
Donovan WH, Dimitrijevic MR, Dahm L, Dimitrijevic M . Neurophysiological approaches to chronic pain following spinal cord injury. Paraplegia 1982; 20: 135–146.
Riddoch G . The clinical features of central pain. Lancet 1938; 234: 1150–1156.
Siddall PJ, Yezierski RP, Loeser JD . Pain following spinal cord injury: clinical features, prevalence, and taxonomy. IASP Newsletter 2000; 3: 3–7.
Bryce TN, Biering-Sorensen F, Finnerup NB et al. International spinal cord injury pain (ISCIP) classification: Part 2. initial validation using vignettes. Spinal Cord, (submitted).
Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS et al. A new definition of neuropathic pain. Pain 2011; 152: 2204–2205.
Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26 (Suppl 1): S50–S56.
Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D . Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 2008; 138: 343–353.
Putzke JD, Richards JS, Hicken BL, Ness TJ, Kezar L, DeVivo M . Pain classification following spinal cord injury: the utility of verbal descriptors. Spinal Cord 2002; 40: 118–127.
Defrin R, Ohry A, Blumen N, Urca G . Characterization of chronic pain and somatosensory function in spinal cord injury subjects. Pain 2001; 89: 253–263.
Costigan M, Scholz J, Woolf CJ . Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32.
Widerstrom-Noga E, Biering-Sorensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP et al. The international spinal cord injury pain basic data set. Spinal Cord 2008; 46: 818–823.
Merskey H, Bogduk N . Classification of chronic pain. In: Merskey H, Bogduk N, (eds). Part III: Pain Terms, a Current List with Definitions and Notes on Usage. IASP Task Force on Taxonomy. IASP Press: Seattle, 1994, 209–214.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Development of the International Spinal Cord Injury Pain Classification was made possible by a grant from the Paralyzed Veterans of America, Washington DC to Mount Sinai School of Medicine.
Rights and permissions
About this article
Cite this article
Bryce, T., Biering-Sørensen, F., Finnerup, N. et al. International Spinal Cord Injury Pain Classification: part I. Background and description. Spinal Cord 50, 413–417 (2012). https://doi.org/10.1038/sc.2011.156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.156
Keywords
This article is cited by
-
The impact of time from injury to surgery on the risk of neuropathic pain after traumatic spinal cord injury
Journal of Orthopaedic Surgery and Research (2023)
-
The international spinal cord injury pain basic data set (version 3.0)
Spinal Cord (2023)
-
Neuropathic Pain and Spinal Cord Injury: Management, Phenotypes, and Biomarkers
Drugs (2023)
-
Efficacy of transcutaneous electrical nerve stimulation in people with pain after spinal cord injury: a meta-analysis
Spinal Cord (2022)
-
Cross-cultural adaptation and validation of the French version of the Spinal Cord Injury Pain Instrument (SCIPI)
Spinal Cord (2022)