Abstract
Background:
Metformin is an inhibitor of complex 1 in the respiratory chain, and is widely used to reduce insulin resistance. It has also been described to have pleotropic effects including via AMPK on inhibiting the mTOR kinase. Pre-clinical and epidemiological studies suggest an ability to modulate disease evolution in prostate cancer. In this study, we aimed to (i) demonstrate safety and tolerability of neoadjuvant metformin administration and (ii) document changes in proliferative (Ki67) and AMPK-related signalling indices between matching biopsies and prostatectomies
Methods:
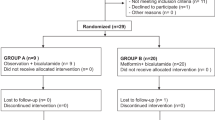
Men were treated in a single-arm ‘window of opportunity’ study between their decision to undergo radical prostatectomy and the operation itself. Forty patients were planned but only 24 patients were enrolled owing to slow accrual. Twenty-one patients were evaluable for pathological outcomes and 22 for serum metabolic indices. Metformin was given at doses to 500 mg t.i.d. Ki67 index was calculated using the Aperio-positive pixel count algorithm, whereas immunohistochemical measurements were by consensus H-Score. Comparative statistics were analysed by students t-tests and/or Wilcoxon matched pairs signed rank test.
Results:
Baseline characteristics included median PSA 6 ng ml−1 (3.22–36.11 ng ml−1). Median duration of drug treatment was 41 days (18–81). Treatment was well tolerated with only three patients developing G3/4 toxicities. In a per patient and per tumour analyses, metformin reduced the Ki67 index by relative amounts of 29.5 and 28.6 % (P=0.0064 and P=0.0042) respectively. There was also a significant decrease in P-4EBP1 staining (P<0.001) but no change in P-AMPK or P-ACC. There were no correlations between any metabolic, morphometric or cancer-related serum indices. There was a trend towards PSA reduction (P=0.08). The study is limited by small patient numbers and tumour heterogeneity.
Conclusions:
Neoadjuvant metformin is well tolerated prior to radical prostatectomy. Data to date indicate promising effects on metabolic and tissue proliferation and signalling parameters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Society AC. Global Cancer Facts & Figures, 2nd edn. (Society AC), 2011.
Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27: 3576–3586.
Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG . Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer 2008; 8: 501–505.
Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG . Increased serum insulin associated with increased risk of prostate cancer recurrence. Prostate 2002; 50: 1–3.
Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG . Serum insulin level, disease stage, prostate specific antigen (PSA) and Gleason score in prostate cancer. Br J Cancer 2002; 87: 726–728.
Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008; 9: 1039–1047.
Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E et al. Insulin receptor expression by human prostate cancers. Prostate 2009; 69: 33–40.
Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A . Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 2013; 8: e71583.
Qiu H, Rhoads GG, Berlin JA, Marcella SW, Demissie K . Initial metformin or sulphonylurea exposure and cancer occurrence among patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15: 349–357.
Margel D, Urbach D, Lipscombe LL, Bell CM, Kulkarni G, Austin PC et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst 2013; 105: 1123–1131.
Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ . Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013 Apr; 63: 709–716.
Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 2013; 31: 3069–3075.
Niraula S, Dowling RJ, Ennis M, Chang MC, Done SJ, Hood N et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 2012; 135: 821–830.
Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncology 2009; 27: 3297–3302.
Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 2011; 128: 783–794.
Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol 2012; 30: 2593–2600.
Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011; 71: 4366–4372.
Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 2012; 15: 346–352.
Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB . Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors 2007; 25: 209–226.
Manning BD . Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 2004; 167: 399–403.
Stambolic V, Woodgett JR, Fantus IG, Pritchard KI, Goodwin PJ . Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Res Treatment 2009; 114: 387–389.
Owen MR, Doran E, Halestrap AP . Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. J Biochem 2000; 348 (Pt 3): 607–614.
Obinata D, Takayama K, Urano T, Murata T, Kumagai J, Fujimura T et al. Oct1 regulates cell growth of LNCaP cells and is a prognostic factor for prostate cancer. Int J Cancer 2012; 130: 1021–1028.
Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M et al. Towards a knowledge-based human protein atlas. Nat Biotechnol 2010; 28: 1248–1250.
Verhoven B, Yan Y, Ritter M, Khor LY, Hammond E, Jones C et al. Ki-67 is an independent predictor of metastasis and cause-specific mortality for prostate cancer patients treated on Radiation Therapy Oncology Group (RTOG) 94-08. Int J Radiat Oncol Biol Phys 2013; 86: 317–323.
Bubendorf L, Sauter G, Moch H, Schmid HP, Gasser TC, Jordan P et al. Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol 1996; 178: 437–441.
Zellweger T, Gunther S, Zlobec I, Savic S, Sauter G, Moch H et al. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer 2009; 124: 2116–2123.
Bubendorf L, Tapia C, Gasser TC, Casella R, Grunder B, Moch H et al. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum Pathol 1998; 29: 949–954.
Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M et al. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst 2013; 105: 1881–1890.
Rothermundt CA, Cathomas R, Templeton A, Winterhalder RC, Strebel R, Baertschi D, Pollak M, Lui L, Crowe S, Gillessen S (eds). Metformin in Chemotherapy-Naïve Castration Resistant Prostate Cancer (CRPC): A Multicenter Phase II Trial (SAKK 08/09). ESMO: Vienna, 2012.
Acknowledgements
This research was funded in part by the Terry Fox Foundation, Princess Margaret Hospital Foundation and the Jewish General Hospital Foundation. MS was in part supported by the Robert-Bosch Foundation, Stuttgart, Germany, and the IZEPHA Grant, University of Tübingen, Tübingen, Germany
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Joshua, A., Zannella, V., Downes, M. et al. A pilot ‘window of opportunity’ neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dis 17, 252–258 (2014). https://doi.org/10.1038/pcan.2014.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2014.20
This article is cited by
-
The role of autophagy in prostate cancer and prostatic diseases: a new therapeutic strategy
Prostate Cancer and Prostatic Diseases (2024)
-
Is it still worth pursuing the repurposing of metformin as a cancer therapeutic?
British Journal of Cancer (2023)
-
Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study
Molecular Biomedicine (2022)
-
Efficacy of metformin therapy in patients with cancer: a meta-analysis of 22 randomised controlled trials
BMC Medicine (2022)
-
The role of metformin, statins and diet in men on active surveillance for prostate cancer
World Journal of Urology (2022)