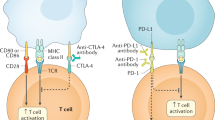
Abstract
Targeting a T-cell inhibitory checkpoint with the anti-CTLA-4 monoclonal antibody, ipilimumab, represents a scientific breakthrough in immunotherapy for the treatment of cancer. However, ipilimumab therapy is also associated with unique side effects, known as immune-related adverse events (irAEs), which need to be recognized and managed with immunosuppressive agents. To date, the majority of our knowledge regarding ipilimumab-associated side effects is based upon clinical studies in melanoma. Here we provide a review of ipilimumab-induced irAEs and our experience in a cohort of 44 patients with prostate cancer who were treated at the MD Anderson Cancer Center on two different clinical trial protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Leach DR, Krummel MF, Allison JP . Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–1736.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800.
Krummel MF, Allison JP . CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182: 459–465.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723.
Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526.
Weber JS, Kahler KC, Hauschild A . Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30: 2691–2697.
Quezada SA, Peggs KS, Curran MA, Allison JP . CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006; 116: 1935–1945.
Fasso M, Waitz R, Hou Y, Rim T, Greenberg NM, Shastri N et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci USA 2008; 105: 3509–3514.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. Eur J Cancer 2013; 49: 24.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712.
Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann NY Acad Sci 2013; 1291: 1–13.
Fecher LA, Agarwala SS, Hodi FS, Weber JS . Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013; 18: 733–743.
Fadel F, El Karoui K, Knebelmann B . Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009; 361: 211–212.
O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 2010; 21: 1712–1717.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11: 155–164.
Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13: 501–508.
van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13: 509–517.
Bristol-Myers Squibb Ipilimumab US prescribing information. http://www.yervoy.com/pdf/pi_yervoy.pdf2013.
Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother 2005; 28: 593–598.
Dillard T, Yedinak CG, Alumkal J, Fleseriu M . Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010; 13: 29–38.
Robinson MR, Chan CC, Yang JC, Rubin BI, Gracia GJ, Sen HN et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother 2004; 27: 478–479.
Sun J, Schiffman J, Raghunath A, Ng Tang D, Chen H, Sharma P . Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun 2008; 8: 9.
Wilgenhof S, Neyns B . Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol 2011; 22: 991–993.
Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013; 8: e53745.
Bhatia S, Huber BR, Upton MP, Thompson JA . Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: a case report. J Immunother 2009; 32: 203–205.
Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 2003; 100: 4712–4717.
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 2003; 100: 8372–8377.
Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol 2005; 23: 741–750.
Jaber SH, Cowen EW, Haworth LR, Booher SL, Berman DM, Rosenberg SA et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol 2006; 142: 166–172.
Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006; 24: 2283–2289.
Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010; 10: 11.
Min L, Vaidya A, Becker C . Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 2011; 164: 303–307.
Delyon J, Mateus C, Lambert T . Hemophilia A induced by ipilimumab. N Engl J Med 2011; 365: 1747–1748.
von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med 2009; 7: 35.
Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210: 1695–1710.
Alegre ML, Shiels H, Thompson CB, Gajewski TF . Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol 1998; 161: 3347–3356.
Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F et al. Cutting edge: CTLA-4—B7 interaction suppresses Th17 cell differentiation. J Immunol 2010; 185: 1375–1378.
Garber K . Anti-IL-17 mAbs herald new options in psoriasis. Nat Biotechnol 2012; 30: 475–477.
Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA 2008; 105: 20410–20415.
Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931.
Klein O, Ebert LM, Nicholaou T, Browning J, Russell SE, Zuber M et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res 2009; 15: 2507–2513.
Tarhini A . Immune-Mediated Adverse Events Associated with Ipilimumab CTLA-4 Blockade Therapy: The Underlying Mechanisms and Clinical Management. Scientifica (Cairo) 2013; 2013: 857519.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JG's work is supported by an American Society of Clinical Oncology Young Investigator Award and an NIH K12 grant. PS’s work is supported by an NIH R01 grant (1-R01 CA1633793-01), a research grant form Cancer Prevention Institute of Texas (RP120108), a Prostate Cancer Foundation Challenge Grant in Immunology and an American Association for Cancer Research/Cancer Research Institute/Stand-Up-To-Cancer Dream Team grant. PS also serves as a consultant for Bristol Myers-Squibb, Glaxo-Smith Kline and MedImmune/Astrazeneca. PS is also a founder and consultant for Jounce Therapeutics. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gao, J., He, Q., Subudhi, S. et al. Review of immune-related adverse events in prostate cancer patients treated with ipilimumab: MD Anderson experience. Oncogene 34, 5411–5417 (2015). https://doi.org/10.1038/onc.2015.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.5
This article is cited by
-
Short Review on Advances in Hydrogel-Based Drug Delivery Strategies for Cancer Immunotherapy
Tissue Engineering and Regenerative Medicine (2022)
-
Immuntherapie beim Prostatakarzinom – aus Alt macht Neu?
Der Urologe (2018)
-
VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer
Nature Medicine (2017)
-
Ipilimumab
Reactions Weekly (2015)