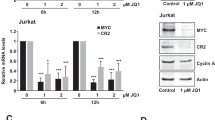
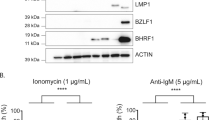
Abstract
Epstein-Barr virus (EBV) has evolved exquisite controls over its host cells, human B lymphocytes, not only directing these cells during latency to proliferate and thereby expand the pool of infected cells, but also to survive and thereby persist for the lifetime of the infected individual. Although these activities ensure the virus is successful, they also make the virus oncogenic, particularly when infected people are immunosuppressed. Here we show, strikingly, that one set of EBV’s microRNAs (miRNAs) both sustain Burkitt’s lymphoma (BL) cells in the absence of other viral oncogenes and promote the transformation of primary B lymphocytes. BL cells were engineered to lose EBV and found to die by apoptosis and could be rescued by constitutively expressing viral miRNAs in them. Two of these EBV miRNAs were found to target caspase 3 to inhibit apoptosis at physiological concentrations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Nanbo A, Sugden A, Sugden B . The coupling of synthesis and partitioning of EBV's plasmid replicon is revealed in live cells. EMBO J 2007; 26: 4252–4262.
Vereide D, Sugden B . Insights into the evolution of lymphomas induced by Epstein-Barr virus. Advances in cancer research 2010; 108: 1–19.
Vereide DT, Sugden B . Lymphomas differ in their dependence on Epstein-Barr virus. Blood 2011; 117: 1977–1985.
Kirchmaier AL, Sugden B . Dominant-negative inhibitors of EBNA-1 of Epstein-Barr virus. J Virol 1997; 71: 1766–1775.
Kelly G, Bell A, Rickinson A . Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nat Med 2002; 8: 1098–1104.
Kuzembayeva M, Chiu YF, Sugden B . Comparing proteomics and RISC immunoprecipitations to identify targets of Epstein-Barr viral miRNAs. PLoS One 2012; 7: e47409.
Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF et al. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med 2008; 205: 2551–2560.
Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008; 132: 875–886.
Oliver PM, Vass T, Kappler J, Marrack P . Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med 2006; 203: 731–741.
Anderton E, Yee J, Smith P, Crook T, White RE, Allday MJ . Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt's lymphoma. Oncogene 2008; 27: 421–433.
Clybouw C, McHichi B, Mouhamad S, Auffredou MT, Bourgeade MF, Sharma S et al. EBV infection of human B lymphocytes leads to down-regulation of Bim expression: relationship to resistance to apoptosis. J Immunol 2005; 175: 2968–2973.
Paschos K, Smith P, Anderton E, Middeldorp JM, White RE, Allday MJ . Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog 2009; 5: e1000492.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233.
Rajewsky N . microRNA target predictions in animals. Nat Genet 2006; 38 (Suppl): S8–S13.
Grundhoff A, Sullivan CS, Ganem D . A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA 2006; 12: 733–750.
Schafer A, Cai X, Bilello JP, Desrosiers RC, Cullen BR . Cloning and analysis of microRNAs encoded by the primate gamma-herpesvirus rhesus monkey rhadinovirus. Virology 2007; 364: 21–27.
Walz N, Christalla T, Tessmer U, Grundhoff A . A global analysis of evolutionary conservation among known and predicted gammaherpesvirus microRNAs. J Virol 2010; 84: 716–728.
Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E . Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol 1993; 67: 2209–2220.
Riley KJ, Rabinowitz GS, Yario TA, Luna JM, Darnell RB, Steitz JA . EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. EMBO J 2012; 31: 2207–2221.
Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M et al. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog 2012; 8: e1002484.
Dolken L, Malterer G, Erhard F, Kothe S, Friedel CC, Suffert G et al. Systematic analysis of viral and cellular microRNA targets in cells latently infected with human gamma-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe 2010; 7: 324–334.
Niwa H, Yamamura K, Miyazaki J . Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108: 193–199.
Seto E, Moosmann A, Gromminger S, Walz N, Grundhoff A, Hammerschmidt W . Micro RNAs of Epstein-Barr virus promote cell cycle progression and prevent apoptosis of primary human B cells. PLoS Pathogens 2010; 6: e1001063.
Suffert G, Malterer G, Hausser J, Viiliainen J, Fender A, Contrant M et al. Kaposi's sarcoma herpesvirus microRNAs target caspase 3 and regulate apoptosis. PLoS Pathogens 2011; 7: e1002405.
Lee DY, Sugden B . The LMP1 oncogene of EBV activates PERK and the unfolded protein response to drive its own synthesis. Blood 2008; 111: 2280–2289.
Gaillard C, Strauss F . Ethanol precipitation of DNA with linear polyacrylamide as carrier. Nucleic Acids Res 1990; 18: 378.
Pratt ZL, Kuzembayeva M, Sengupta S, Sugden B . The microRNAs of Epstein-Barr Virus are expressed at dramatically differing levels among cell lines. Virology 2009; 386: 387–397.
Pratt ZL, Zhang J, Sugden B . The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. J Virol 2012; 86: 4380–4393.
Pruitt KD, Tatusova T, Klimke W, Maglott DR . NCBI reference sequences: current status, policy and new initiatives. Nucleic Acids Res 2009; 37: D32–D36.
Altmann M, Hammerschmidt W . Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol 2005; 3: e404.
Acknowledgements
We thank Alan Rickinson for kindly providing the early passage BL cell lines and Dagmar Pich for her invaluable support. This work was funded by grants from the National Cancer Institute, National Institutes of Health (grant P01 CA022443, grants R01 CA133027 and R01 CA070723) and Deutsche Forschungsgemeinschaft (grants SFB455 and SFBTR5). David Vereide was supported by a predoctoral fellowship from the National Cancer Center. Takanobu Tagawa is supported by a predoctoral grant of DAAD, German Academic Exchange Service. Bill Sugden is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Vereide, D., Seto, E., Chiu, YF. et al. Epstein–Barr virus maintains lymphomas via its miRNAs. Oncogene 33, 1258–1264 (2014). https://doi.org/10.1038/onc.2013.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.71
Keywords
This article is cited by
-
Exploring the link between viruses and cancer in companion animals: a comprehensive and comparative analysis
Infectious Agents and Cancer (2023)
-
Burkitt lymphoma
Nature Reviews Disease Primers (2022)
-
Latency and lytic replication in Epstein–Barr virus-associated oncogenesis
Nature Reviews Microbiology (2019)
-
Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis
Medical Microbiology and Immunology (2019)
-
Coordinated repression of BIM and PUMA by Epstein–Barr virus latent genes maintains the survival of Burkitt lymphoma cells
Cell Death & Differentiation (2018)