Abstract
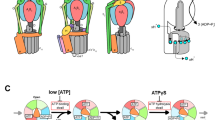
F1-ATPase is a rotary molecular motor in which unidirectional rotation of the central γ subunit is powered by ATP hydrolysis in three catalytic sites arranged 120° apart around γ. To study how hydrolysis reactions produce mechanical rotation, we observed rotation under an optical microscope to see which of the three sites bound and released a fluorescent ATP analog. Assuming that the analog mimics authentic ATP, the following scheme emerges: (i) in the ATP-waiting state, one site, dictated by the orientation of γ, is empty, whereas the other two bind a nucleotide; (ii) ATP binding to the empty site drives an ∼80° rotation of γ; (iii) this triggers a reaction(s), hydrolysis and/or phosphate release, but not ADP release in the site that bound ATP one step earlier; (iv) completion of this reaction induces further ∼40° rotation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abrahams, J.P., Leslie, A.G., Lutter, R. & Walker, J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 (1994).
Boyer, P.D. The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochim. Biophys. Acta 1140, 215–250 (1993).
Boyer, P.D. The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem. 66, 717–749 (1997).
Yoshida, M., Muneyuki, E. & Hisabori, T. ATP synthase—a marvelous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2, 669–677 (2001).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Jr. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Noji, H. et al. Purine but not pyrimidine nucleotides support rotation of F1-ATPase. J. Biol. Chem. 276, 25480–25486 (2001).
Yasuda, R., Noji, H., Kinosita, K. Jr. & Yoshida, M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell 93, 1117–1124 (1998).
Adachi, K. et al. Stepping rotation of F1-ATPase visualized through angle-resolved single-fluorophore imaging. Proc. Natl. Acad. Sci. USA 97, 7243–7247 (2000).
Boyer, P.D. Catalytic site forms and controls in ATP synthase catalysis. Biochim. Biophys. Acta 1458, 252–262 (2000).
Kinosita, K. Jr., Yasuda, R., Noji, H. & Adachi, K. A rotary molecular motor that can work at near 100% efficiency. Phil. Trans. R. Soc. Lond. B 355, 473–489 (2000).
Weber, J. & Senior, A.E. ATP synthase: what we know about ATP hydrolysis and what we do not know about ATP synthesis. Biochim. Biophys. Acta 1458, 300–309 (2000).
Yasuda, R., Noji, H., Yoshida, M., Kinosita, K. Jr. & Itoh, H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature 410, 898–904 (2001).
Oiwa, K. et al. Comparative single-molecule and ensemble myosin enzymology: sulfoindocyanine ATP and ADP derivatives. Biophys. J. 78, 3048–3071 (2000).
Tokunaga, M., Kitamura, K., Saito, K., Iwane, A.H. & Yanagida, T. Single molecule imaging of fluorophores and enzymatic reactions achieved by objective-type total internal reflection fluorescence microscopy. Biochem. Biophys. Res. Commun. 235, 47–53 (1997).
Funatsu, T., Harada, Y., Tokunaga, M., Saito, K. & Yanagida, T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559 (1995).
Ishijima, A. et al. Simultaneous observation of individual ATPase and mechanical events by a single myosin molecule during interaction with actin. Cell 92, 161–171 (1998).
Ha, T., Enderle, T., Chemla, S., Selvin, R. & Weiss, S. Single molecule dynamics studied by polarization modulation. Phys. Rev. Lett. 77, 3979–3982 (1996).
Sase, I., Miyata, H., Ishiwata, S. & Kinosita, K. Jr. Axial rotation of sliding actin filaments revealed by single-fluorophore imaging. Proc. Natl. Acad. Sci. USA 94, 5646–5650 (1997).
Axelrod, D. Total internal reflection fluorescence at biological surfaces. in Noninvasive Techniques in Cell Biology (eds. Foskett, J.K. & Grinstein, S.) 93–127 (Wiley-Liss, New York, 1990).
Jault, J.M. et al. The α3β3γ subcomplex of the F1-ATPase from the thermophilic bacillus PS3 with the βT165S substitution does not entrap inhibitory MgADP in a catalytic site during turnover. J. Biol. Chem. 271, 28818–28824 (1996).
Matsui, T. et al. Catalytic activity of the α3β3γ complex of F1-ATPase without noncatalytic nucleotide binding site. J. Biol. Chem. 272, 8215–8221 (1997).
Hirono-Hara, Y. et al. Pause and rotation of F1-ATPase during catalysis. Proc. Natl. Acad. Sci. USA 98, 13649–13654 (2001).
Vale, R.D. & Oosawa, F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv. Biophys. 26, 97–134 (1990).
Astumian, R.D. & Bier, M. Fluctuation driven ratchets: molecular motors. Phys. Rev. Lett. 72, 1766–1769 (1994).
Hunt, A.J., Gittes, F. & Howard, J. The force exerted by a single kinesin molecule against a viscous load. Biophys. J. 67, 766–781 (1994).
Masaike, T., Muneyuki, E., Noji, H., Kinosita, K. Jr. & Yoshida, M. F1-ATPase changes its conformations upon phosphate release. J. Biol. Chem. 277, 21643–21649 (2002).
Shimabukuro, K. et al. Catalysis and rotation of F1 motor: cleavage of ATP at the catalytic site occurs in 1 ms before 40° substep rotation. Proc. Natl. Acad. Sci. USA 100, 14731–14736 (2003).
Weber, J. & Senior, A.E. Bi-site catalysis in F1-ATPase: does it exist? J. Biol. Chem. 276, 35422–35428 (2001).
Boyer, P.D. Catalytic site occupancy during ATP synthase catalysis. FEBS Lett. 512, 29–32 (2002).
Masaike, T. et al. Rotation of F1-ATPase and the hinge residues of the β subunit. J. Exp. Biol. 203, 1–8 (2000).
Dou, C., Fortes, P.A. & Allison, W.S. The α3(βY341W)3 γ subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 fails to dissociate ADP when MgATP is hydrolyzed at a single catalytic site and attains maximal velocity when three catalytic sites are saturated with MgATP. Biochemistry 37, 16757–16764 (1998).
Ren, H. & Allison, W.S. Substitution of betaGlu201 in the α3β3γ subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 increases the affinity of catalytic sites for nucleotides. J. Biol. Chem. 275, 10057–10063 (2000).
Mitome, N. et al. The presence of phosphate at a catalytic site suppresses the formation of the MgADP-inhibited form of F1-ATPase. Eur. J. Biochem. 269, 53–60 (2002).
Nishizaka, T., Seo, R., Tadakuma, H., Kinosita, K. Jr. & Ishiwata, S. Characterization of single actomyosin rigor bonds: load dependence of lifetime and mechanical properties. Biophys. J. 79, 962–974 (2000).
Nishizaka, T., Miyata, H., Yoshikawa, H., Ishiwata, S. & Kinosita, K. Jr. Unbinding force of a single motor molecule of muscle measured using optical tweezers. Nature 377, 251–254 (1995).
Acknowledgements
We thank R. Yasuda, K. Adachi and H. Itoh for technical assistance and critical discussion; R. Nakamori and Y. Funamoto for technical assistance; D.R. Trentham for initiation of our collaboration; T. Masaike, B. Brenner and H. Kojima for critical discussion; M. Shio, K. Abe and I. Sase for the microscope techniques; and M. Uno and H. Umezawa for management of laboratories and collaboration. This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a Core Research for Evolutional Science and Technology (CREST) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Nishizaka, T., Oiwa, K., Noji, H. et al. Chemomechanical coupling in F1-ATPase revealed by simultaneous observation of nucleotide kinetics and rotation. Nat Struct Mol Biol 11, 142–148 (2004). https://doi.org/10.1038/nsmb721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb721
This article is cited by
-
Direct visualization of virus removal process in hollow fiber membrane using an optical microscope
Scientific Reports (2021)
-
Angle change of the A-domain in a single SERCA1a molecule detected by defocused orientation imaging
Scientific Reports (2021)
-
Correlation between the numbers of rotation steps in the ATPase and proton-conducting domains of F- and V-ATPases
Biophysical Reviews (2020)
-
Reconstitution of FoF1-ATPase-based biomimetic systems
Nature Reviews Chemistry (2019)
-
Single-molecule pull-out manipulation of the shaft of the rotary motor F1-ATPase
Scientific Reports (2019)