Abstract
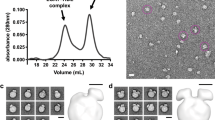
The cardioprotective function of high-density lipoprotein (HDL) is largely attributed to its ability to facilitate transport of cholesterol from peripheral tissues to the liver. However, HDL may become dysfunctional through oxidative modification, impairing cellular cholesterol efflux. Here we report a refined molecular model of nascent discoidal HDL, determined using hydrogen-deuterium exchange mass spectrometry. The model reveals two apolipoprotein A1 (apoA1) molecules arranged in an antiparallel double-belt structure, with residues 159–180 of each apoA1 forming a protruding solvent-exposed loop. We further show that this loop, including Tyr166, a preferred target for site-specific oxidative modification within atheroma, directly interacts with and activates lecithin cholesterol acyl transferase. These studies identify previously uncharacterized structural features of apoA1 in discoidal HDL that are crucial for particle maturation, and elucidate a structural and molecular mechanism for generating a dysfunctional form of HDL in atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Change history
27 February 2008
In the version of this article initially published, Figures 2c,d,e and 5 showed an incorrect model for discoidal HDL, with the apoA1 molecules in a clockwise orientation, in contrast with their counterclockwise orientation in the final mode described in the text. The correct model is deposited under the same accession number, PM0074956, at http://mi.caspur.it/PMDB/main.php. The error has been corrected in the PDF and HTML versions of the article.
22 May 2008
In the version of this article initially published, the legend text for Figure 2c was incorrect, with the terms “clockwise” and “counterclockwise” swapped. The error has been corrected in the PDF and HTML versions of the article.
References
Barter, P.J. & Rye, K.A. Relationship between the concentration and antiatherogenic activity of high-density lipoproteins. Curr. Opin. Lipidol. 17, 399–403 (2006).
Rader, D.J. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat. Clin. Pract. Cardiovasc. Med. 4, 102–109 (2007).
Assmann, G. & Gotto, A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation 109, III8–III14 (2004).
Plump, A.S., Scott, C.J. & Breslow, J.L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA 91, 9607–9611 (1994).
Nissen, S.E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J. Am. Med. Assoc. 290, 2292–2300 (2003).
Linsel-Nitschke, P. & Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4, 193–205 (2005).
Ansell, B.J. et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108, 2751–2756 (2003).
Nicholls, S.J., Zheng, L. & Hazen, S.L. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 15, 212–219 (2005).
Kontush, A. & Chapman, M.J. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58, 342–374 (2006).
Tall, A.R. CETP inhibitors to increase HDL cholesterol levels. N. Engl. J. Med. 356, 1364–1366 (2007).
Nissen, S.E. et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356, 1304–1316 (2007).
Kastelein, J.J. et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 356, 1620–1630 (2007).
Zheng, L. et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114, 529–541 (2004).
Peng, D.Q. et al. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J. Biol. Chem. 280, 33775–33784 (2005).
Zheng, L. et al. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J. Biol. Chem. 280, 38–47 (2005).
Bergt, C. et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 101, 13032–13037 (2004).
Shao, B. et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J. Biol. Chem. 280, 5983–5993 (2005).
Ajees, A.A., Anantharamaiah, G.M., Mishra, V.K., Hussain, M.M. & Murthy, H.M. Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases. Proc. Natl. Acad. Sci. USA 103, 2126–2131 (2006).
Li, Y., Kijac, A.Z., Sligar, S.G. & Rienstra, C.M. Structural analysis of nanoscale self-assembled discoidal lipid bilayers by solid-state NMR spectroscopy. Biophys. J. 91, 3819–3828 (2006).
Segrest, J.P. et al. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 274, 31755–31758 (1999).
Maiorano, J.N., Jandacek, R.J., Horace, E.M. & Davidson, W.S. Identification and structural ramifications of a hinge domain in apolipoprotein A-I discoidal high-density lipoproteins of different size. Biochemistry 43, 11717–11726 (2004).
Silva, R.A., Hilliard, G.M., Li, L., Segrest, J.P. & Davidson, W.S. A mass spectrometric determination of the conformation of dimeric apolipoprotein A-I in discoidal high density lipoproteins. Biochemistry 44, 8600–8607 (2005).
Bhat, S., Sorci-Thomas, M.G., Alexander, E.T., Samuel, M.P. & Thomas, M.J. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J. Biol. Chem. 280, 33015–33025 (2005).
Thomas, M.J., Bhat, S. & Sorci-Thomas, M.G. The use of chemical cross-linking and mass spectrometry to elucidate the tertiary conformation of lipid-bound apolipoprotein A-I. Curr. Opin. Lipidol. 17, 214–220 (2006).
Zannis, V.I., Chroni, A. & Krieger, M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 84, 276–294 (2006).
Alexander, E.T. et al. Apolipoprotein A-I helix 6 negatively charged residues attenuate lecithin-cholesterol acyltransferase (LCAT) reactivity. Biochemistry 44, 5409–5419 (2005).
Mishra, V.K. et al. Association of a model class A (apolipoprotein) amphipathic alpha helical peptide with lipid: high resolution NMR studies of peptide.lipid discoidal complexes. J. Biol. Chem. 281, 6511–6519 (2006).
Minnich, A. et al. Site-directed mutagenesis and structure-function analysis of the human apolipoprotein A-I. Relation between lecithin-cholesterol acyltransferase activation and lipid binding. J. Biol. Chem. 267, 16553–16560 (1992).
Sorci-Thomas, M.G., Curtiss, L., Parks, J.S., Thomas, M.J. & Kearns, M.W. Alteration in apolipoprotein A-I 22-mer repeat order results in a decrease in lecithin:cholesterol acyltransferase reactivity. J. Biol. Chem. 272, 7278–7284 (1997).
Banka, C.L., Bonnet, D.J., Black, A.S., Smith, R.S. & Curtiss, L.K. Localization of an apolipoprotein A-I epitope critical for activation of lecithin-cholesterol acyltransferase. J. Biol. Chem. 266, 23886–23892 (1991).
Borhani, D.W., Rogers, D.P., Engler, J.A. & Brouillette, C.G. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA 94, 12291–12296 (1997).
Rogers, D.P. et al. Truncation of the amino terminus of human apolipoprotein A-I substantially alters only the lipid-free conformation. Biochemistry 36, 288–300 (1997).
Martin, D.D., Budamagunta, M.S., Ryan, R.O., Voss, J.C. & Oda, M.N. Apolipoprotein A-I assumes a “looped belt” conformation on reconstituted high density lipoprotein. J. Biol. Chem. 281, 20418–20426 (2006).
Furbee, J.W., Jr., Sawyer, J.K. & Parks, J.S. Lecithin:cholesterol acyltransferase deficiency increases atherosclerosis in the low density lipoprotein receptor and apolipoprotein E knockout mice. J. Biol. Chem. 277, 3511–3519 (2002).
Lambert, G. et al. Analysis of glomerulosclerosis and atherosclerosis in lecithin cholesterol acyltransferase-deficient mice. J. Biol. Chem. 276, 15090–15098 (2001).
Lee, R.G. et al. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95, 998–1004 (2004).
Hovingh, G.K. et al. Compromised LCAT function is associated with increased atherosclerosis. Circulation 112, 879–884 (2005).
Hovingh, G.K. et al. Inherited disorders of HDL metabolism and atherosclerosis. Curr. Opin. Lipidol. 16, 139–145 (2005).
Matz, C.E. & Jonas, A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 257, 4535–4540 (1982).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Chisholm, J.W., Gebre, A.K. & Parks, J.S. Characterization of C-terminal histidine-tagged human recombinant lecithin:cholesterol acyltransferase. J. Lipid Res. 40, 1512–1519 (1999).
Parks, J.S., Gebre, A.K. & Furbee, J.W. Lecithin-cholesterol acyltransferase. Assay of cholesterol esterification and phospholipase A2 activities. Methods Mol. Biol. 109, 123–131 (1999).
Acknowledgements
This work was supported by US National Institutes of Health grants P01 HL076491, P50 HL77107, HL70621, HL066082, 1R15 GM070469-01, P01 HL049373 and HL 054176, and the Cleveland Clinic Foundation General Clinical Research Center (M01 RR018390). Z.W. was partially supported by an American Heart Association Fellowship Award.
Author information
Authors and Affiliations
Contributions
Z.W. and S.L.H. designed all studies and prepared the manuscript. Z.W. carried out all biochemical studies. V.G., M.A.W. and J.M.S. performed computational modeling. J.D.S. and J.S.P. assisted in generation of recombinant proteins. L.Z. and Z.W. performed all proteomic studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Tables 1 and 2, Supplementary Methods (PDF 757 kb)
Rights and permissions
About this article
Cite this article
Wu, Z., Wagner, M., Zheng, L. et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol 14, 861–868 (2007). https://doi.org/10.1038/nsmb1284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1284
This article is cited by
-
Changes in high-density lipoprotein cholesterol with risk of Cardiovascular Disease among initially high-density lipoprotein-high participants
Cardiovascular Diabetology (2023)
-
Structural analysis of lecithin:cholesterol acyltransferase bound to high density lipoprotein particles
Communications Biology (2020)
-
Cross-linking/mass spectrometry at the crossroads
Analytical and Bioanalytical Chemistry (2020)
-
The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk
Lipids in Health and Disease (2017)
-
Immunochemical Approach for Monitoring of Structural Transition of ApoA-I upon HDL Formation Using Novel Monoclonal Antibodies
Scientific Reports (2017)