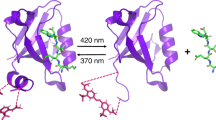
Abstract
Allosteric interactions are typically considered to proceed through a series of discrete changes in bonding interactions that alter the protein conformation. Here we show that allostery can be mediated exclusively by transmitted changes in protein motions. We have characterized the negatively cooperative binding of cAMP to the dimeric catabolite activator protein (CAP) at discrete conformational states. Binding of the first cAMP to one subunit of a CAP dimer has no effect on the conformation of the other subunit. The dynamics of the system, however, are modulated in a distinct way by the sequential ligand binding process, with the first cAMP partially enhancing and the second cAMP completely quenching protein motions. As a result, the second cAMP binding incurs a pronounced conformational entropic penalty that is entirely responsible for the observed cooperativity. The results provide strong support for the existence of purely dynamics-driven allostery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Accession codes
References
Hardy, J.A. & Wells, J.A. Searching for new allosteric sites in enzymes. Curr. Opin. Struct. Biol. 14, 706–715 (2004).
Gao, Z.G. & Jacobson, K.A. Allosterism in membrane receptors. Drug Discov. Today 11, 191–202 (2006).
Swain, J.F. & Gierasch, L.M. The changing landscape of protein allostery. Curr. Opin. Struct. Biol. 16, 102–108 (2006).
Suel, G.M., Lockless, S.W., Wall, M.A. & Ranganathan, R. Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nat. Struct. Biol. 10, 59–69 (2003).
Koshland, D.E., Jr. Conformational changes: how small is big enough? Nat. Med. 4, 1112–1114 (1998).
Changeux, J.P. & Edelstein, S.J. Allosteric mechanisms of signal transduction. Science 308, 1424–1428 (2005).
Bray, D. & Duke, T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu. Rev. Biophys. Biomol. Struct. 33, 53–73 (2004).
Wand, A.J. Dynamic activation of protein function: a view emerging from NMR spectroscopy. Nat. Struct. Biol. 8, 926–931 (2001).
Homans, S.W. Probing the binding entropy of ligand-protein interactions by NMR. ChemBioChem 6, 1585–1591 (2005).
Cooper, A. & Dryden, D.T. Allostery without conformational change. A plausible model. Eur. Biophys. J. 11, 103–109 (1984).
Freire, E. The propagation of binding interactions to remote sites in proteins: analysis of the binding of the monoclonal antibody D1.3 to lysozyme. Proc. Natl. Acad. Sci. USA 96, 10118–10122 (1999).
Pan, H., Lee, J.C. & Hilser, V.J. Binding sites in Escherichia coli dihydrofolate reductase communicate by modulating the conformational ensemble. Proc. Natl. Acad. Sci. USA 97, 12020–12025 (2000).
Kern, D. & Zuiderweg, E.R. The role of dynamics in allosteric regulation. Curr. Opin. Struct. Biol. 13, 748–757 (2003).
Stevens, S.Y., Sanker, S., Kent, C. & Zuiderweg, E.R. Delineation of the allosteric mechanism of a cytidylyltransferase exhibiting negative cooperativity. Nat. Struct. Biol. 8, 947–952 (2001).
Maler, L., Blankenship, J., Rance, M. & Chazin, W.J. Site-site communication in the EF-hand Ca2+-binding protein calbindin D9k. Nat. Struct. Biol. 7, 245–250 (2000).
Lee, A.L., Kinnear, S.A. & Wand, A.J. Redistribution and loss of side chain entropy upon formation of a calmodulin-peptide complex. Nat. Struct. Biol. 7, 72–77 (2000).
Fuentes, E.J., Der, C.J. & Lee, A.L. Ligand-dependent dynamics and intramolecular signaling in a PDZ domain. J. Mol. Biol. 335, 1105–1115 (2004).
Koshland, D.E., Jr. The structural basis of negative cooperativity: receptors and enzymes. Curr. Opin. Struct. Biol. 6, 757–761 (1996).
Brown, A.M. & Crothers, D.M. Modulation of the stability of a gene-regulatory protein dimer by DNA and cAMP. Proc. Natl. Acad. Sci. USA 86, 7387–7391 (1989).
Harman, J.G. Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta 1547, 1–17 (2001).
Passner, J.M., Schultz, S.C. & Steitz, T.A. Modeling the cAMP-induced allosteric transition using the crystal structure of CAP-cAMP at 2.1 A resolution. J. Mol. Biol. 304, 847–859 (2000).
Heyduk, E., Heyduk, T. & Lee, J.C. Intersubunit communications in Escherichia coli cyclic AMP receptor protein: studies of the ligand binding domain. Biochemistry 31, 3682–3688 (1992).
Akke, M. NMR methods for characterizing microsecond to millisecond dynamics in recognition and catalysis. Curr. Opin. Struct. Biol. 12, 642–647 (2002).
Volkman, B.F., Lipson, D., Wemmer, D.E. & Kern, D. Two-state allosteric behavior in a single-domain signaling protein. Science 291, 2429–2433 (2001).
Kalodimos, C.G. et al. Structure and flexibility adaptation in nonspecific and specific protein-DNA complexes. Science 305, 386–389 (2004).
Keramisanou, D. et al. Disorder-order folding transitions underlie catalysis in the helicase motor of SecA. Nat. Struct. Mol. Biol. 13, 594–602 (2006).
Mulder, F.A., Mittermaier, A., Hon, B., Dahlquist, F.W. & Kay, L.E. Studying excited states of proteins by NMR spectroscopy. Nat. Struct. Biol. 8, 932–935 (2001).
Forman-Kay, J.D. The 'dynamics' in the thermodynamics of binding. Nat. Struct. Biol. 6, 1086–1087 (1999).
Cavanagh, J. & Akke, M. May the driving force be with you–whatever it is. Nat. Struct. Biol. 7, 11–13 (2000).
Zidek, L., Novotny, M.V. & Stone, M.J. Increased protein backbone conformational entropy upon hydrophobic ligand binding. Nat. Struct. Biol. 6, 1118–1121 (1999).
Stone, M.J. NMR relaxation studies of the role of conformational entropy in protein stability and ligand binding. Acc. Chem. Res. 34, 379–388 (2001).
Igumenova, T.I., Frederick, K.K. & Wand, A.J. Characterization of the fast dynamics of protein amino acid side chains using NMR relaxation in solution. Chem. Rev. 106, 1672–1699 (2006).
Hilser, V.J., Garcia-Moreno, E.B., Oas, T.G., Kapp, G. & Whitten, S.T. A statistical thermodynamic model of the protein ensemble. Chem. Rev. 106, 1545–1558 (2006).
Gekko, K., Obu, N., Li, J. & Lee, J.C. A linear correlation between the energetics of allosteric communication and protein flexibility in the Escherichia coli cyclic AMP receptor protein revealed by mutation-induced changes in compressibility and amide hydrogen-deuterium exchange. Biochemistry 43, 3844–3852 (2004).
Englander, J.J., Louie, G., McKinnie, R.E. & Englander, S.W. Energetic components of the allosteric machinery in hemoglobin measured by hydrogen exchange. J. Mol. Biol. 284, 1695–1706 (1998).
Yang, D. & Kay, L.E. Contributions to conformational entropy arising from bond vector fluctuations measured from NMR-derived order parameters: application to protein folding. J. Mol. Biol. 263, 369–382 (1996).
Bracken, C., Carr, P.A., Cavanagh, J. & Palmer, A.G., III Temperature dependence of intramolecular dynamics of the basic leucine zipper of GCN4: implications for the entropy of association with DNA. J. Mol. Biol. 285, 2133–2146 (1999).
Gunasekaran, K., Ma, B. & Nussinov, R. Is allostery an intrinsic property of all dynamic proteins? Proteins 57, 433–443 (2004).
Anderson, A.C., O'Neil, R.H., DeLano, W.L. & Stroud, R.M. The structural mechanism for half-the-sites reactivity in an enzyme, thymidylate synthase, involves a relay of changes between subunits. Biochemistry 38, 13829–13836 (1999).
Leslie, A.G. & Wonacott, A.J. Structural evidence for ligand-induced sequential conformational changes in glyceraldehyde 3-phosphate dehydrogenase. J. Mol. Biol. 178, 743–772 (1984).
Hampele, I.C. et al. Structure and function of the dihydropteroate synthase from Staphylococcus aureus. J. Mol. Biol. 268, 21–30 (1997).
Lee, A.L. & Wand, A.J. Microscopic origins of entropy, heat capacity and the glass transition in proteins. Nature 411, 501–504 (2001).
Evenas, J. et al. Ligand-induced structural changes to maltodextrin-binding protein as studied by solution NMR spectroscopy. J. Mol. Biol. 309, 961–974 (2001).
Korzhnev, D.M., Skrynnikov, N.R., Millet, O., Torchia, D.A. & Kay, L.E. An NMR experiment for the accurate measurement of heteronuclear spin-lock relaxation rates. J. Am. Chem. Soc. 124, 10743–10753 (2002).
Farrow, N.A., Zhang, O., Szabo, A., Torchia, D.A. & Kay, L.E. Spectral density function mapping using 15N relaxation data exclusively. J. Biomol. NMR 6, 153–162 (1995).
Lefevre, J.F., Dayie, K.T., Peng, J.W. & Wagner, G. Internal mobility in the partially folded DNA binding and dimerization domains of GAL4: NMR analysis of the N-H spectral density functions. Biochemistry 35, 2674–2686 (1996).
Krizova, H., Zidek, L., Stone, M.J., Novotny, M.V. & Sklenar, V. Temperature-dependent spectral density analysis applied to monitoring backbone dynamics of major urinary protein-I complexed with the pheromone 2-sec-butyl-4,5-dihydrothiazole. J. Biomol. NMR 28, 369–384 (2004).
Acknowledgements
We thank P. Loria (Yale) and P. Huskey (Rutgers) for providing us with scripts for the analysis of some of the relaxation data. This work was supported by US National Science Foundation grant MCB-0618259 to C.G.K. and by US National Institutes of Health grant GM41376 and a Howard Hughes Medical Institute investigatorship to R.H.E.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Molecular drawing of CAPN. (PDF 412 kb)
Supplementary Fig. 2
Classes of chemical shift behavior of CAPN during its sequential interaction with cAMP. (PDF 214 kb)
Supplementary Fig. 3
Relaxation rates of CAPN as a function of the ligation state. (PDF 350 kb)
Supplementary Fig. 4
Overlaid HSQC spectra of CAPN as a function of the ligation state. (PDF 347 kb)
Supplementary Figure 5
Rex values of cAMP1-CAPN at two cAMP concentrations. (PDF 305 kb)
Supplementary Figure 6
Changes in order parameters upon sequential cAMP binding. (PDF 305 kb)
Supplementary Discussion
Contribution to Rex from cAMP binding and dissociation is negligible. (PDF 303 kb)
Rights and permissions
About this article
Cite this article
Popovych, N., Sun, S., Ebright, R. et al. Dynamically driven protein allostery. Nat Struct Mol Biol 13, 831–838 (2006). https://doi.org/10.1038/nsmb1132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1132
This article is cited by
-
Advances of Predicting Allosteric Mechanisms Through Protein Contact in New Technologies and Their Application
Molecular Biotechnology (2023)
-
Allosteric rescue of catalytically impaired ATP phosphoribosyltransferase variants links protein dynamics to active-site electrostatic preorganisation
Nature Communications (2022)
-
Observation of conformational changes that underlie the catalytic cycle of Xrn2
Nature Chemical Biology (2022)
-
Interaction of polyethylene glycol with cytochrome c investigated via in vitro and in silico approaches
Scientific Reports (2021)
-
Unraveling the complex enzymatic machinery making a key galactolipid in chloroplast membrane: a multiscale computer simulation
Scientific Reports (2020)