Abstract
MDM2–MDMX complexes bind the p53 tumor-suppressor protein, inhibiting p53's transcriptional activity and targeting p53 for proteasomal degradation. Inhibitors that disrupt binding between p53 and MDM2 efficiently activate a p53 response, but their use in the treatment of cancers that retain wild-type p53 may be limited by on-target toxicities due to p53 activation in normal tissue. Guided by a novel crystal structure of the MDM2–MDMX–E2(UbcH5B)–ubiquitin complex, we designed MDM2 mutants that prevent E2–ubiquitin binding without altering the RING-domain structure. These mutants lack MDM2's E3 activity but retain the ability to limit p53′s transcriptional activity and allow cell proliferation. Cells expressing these mutants respond more quickly to cellular stress than cells expressing wild-type MDM2, but basal p53 control is maintained. Targeting the MDM2 E3-ligase activity could therefore widen the therapeutic window of p53 activation in tumors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jones, S.N., Roe, A.E., Donehower, L.A. & Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206–208 (1995).
Montes de Oca Luna, R., Wagner, D.S. & Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206 (1995).
Finch, R.A. et al. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62, 3221–3225 (2002).
Migliorini, D. et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 22, 5527–5538 (2002).
Parant, J. et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 29, 92–95 (2001).
Huang, L. et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc. Natl. Acad. Sci. USA 108, 12001–12006 (2011).
Pant, V., Xiong, S., Iwakuma, T., Quintás-Cardama, A. & Lozano, G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proc. Natl. Acad. Sci. USA 108, 11995–12000 (2011).
Moll, U.M. & Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 1, 1001–1008 (2003).
Bista, M., Petrovich, M. & Fersht, A.R. MDMX contains an autoinhibitory sequence element. Proc. Natl. Acad. Sci. USA 110, 17814–17819 (2013).
Jackson, M.W. & Berberich, S.J. MdmX protects p53 from Mdm2-mediated degradation. Mol. Cell. Biol. 20, 1001–1007 (2000).
Stad, R. et al. Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep. 2, 1029–1034 (2001).
Badciong, J.C. & Haas, A.L. MdmX is a RING finger ubiquitin ligase capable of synergistically enhancing Mdm2 ubiquitination. J. Biol. Chem. 277, 49668–49675 (2002).
Iyappan, S. et al. Turning the RING domain protein MdmX into an active ubiquitin-protein ligase. J. Biol. Chem. 285, 33065–33072 (2010).
Minsky, N. & Oren, M. The RING domain of Mdm2 mediates histone ubiquitylation and transcriptional repression. Mol. Cell 16, 631–639 (2004).
Shi, D. & Gu, W. Dual roles of MDM2 in the regulation of p53: ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes Cancer 3, 240–248 (2012).
Momand, J., Zambetti, G.P., Olson, D.C., George, D. & Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237–1245 (1992).
Oliner, J.D. et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 362, 857–860 (1993).
Itahana, K. et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 12, 355–366 (2007).
Uldrijan, S., Pannekoek, W.J. & Vousden, K.H. An essential function of the extreme C-terminus of MDM2 can be provided by MDMX. EMBO J. 26, 102–112 (2007).
Poyurovsky, M.V. et al. The Mdm2 RING domain C-terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J. 26, 90–101 (2007).
Tollini, L.A., Jin, A., Park, J. & Zhang, Y. Regulation of p53 by Mdm2 E3 ligase function is dispensable in embryogenesis and development, but essential in response to DNA damage. Cancer Cell 26, 235–247 (2014).
Khoo, K.H., Verma, C.S. & Lane, D.P. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat. Rev. Drug Discov. 13, 217–236 (2014).
Shangary, S. et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl. Acad. Sci. USA 105, 3933–3938 (2008).
Vassilev, L.T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Ding, Q. et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med. Chem. 56, 5979–5983 (2013).
Andreeff, M. et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin. Cancer Res. 22, 868–876 (2016).
Ray-Coquard, I. et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 13, 1133–1140 (2012).
Linke, K. et al. Structure of the MDM2/MDMX RING domain heterodimer reveals dimerization is required for their ubiquitylation in trans. Cell Death Differ. 15, 841–848 (2008).
Kostic, M., Matt, T., Martinez-Yamout, M.A., Dyson, H.J. & Wright, P.E. Solution structure of the Hdm2 C2H2C4 RING, a domain critical for ubiquitination of p53. J. Mol. Biol. 363, 433–450 (2006).
Dou, H., Buetow, L., Sibbet, G.J., Cameron, K. & Huang, D.T. Essentiality of a non-RING element in priming donor ubiquitin for catalysis by a monomeric E3. Nat. Struct. Mol. Biol. 20, 982–986 (2013).
Plechanovová, A., Jaffray, E.G., Tatham, M.H., Naismith, J.H. & Hay, R.T. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 (2012).
Dou, H., Buetow, L., Sibbet, G.J., Cameron, K. & Huang, D.T. BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 19, 876–883 (2012).
Branigan, E., Plechanovová, A., Jaffray, E.G., Naismith, J.H. & Hay, R.T. Structural basis for the RING-catalyzed synthesis of K63-linked ubiquitin chains. Nat. Struct. Mol. Biol. 22, 597–602 (2015).
Buetow, L. et al. Activation of a primed RING E3-E2-ubiquitin complex by non-covalent ubiquitin. Mol. Cell 58, 297–310 (2015).
Koliopoulos, M.G., Esposito, D., Christodoulou, E., Taylor, I.A. & Rittinger, K. Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity. EMBO J. 35, 1204–1218 (2016).
Sanchez, J.G. et al. Mechanism of TRIM25 catalytic activation in the antiviral RIG-I pathway. Cell Rep. 16, 1315–1325 (2016).
Scott, D.C. et al. Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin-like protein NEDD8. Cell 157, 1671–1684 (2014).
Pruneda, J.N. et al. Structure of an E3:E2∼Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell 47, 933–942 (2012).
Dolezelova, P., Cetkovska, K., Vousden, K.H. & Uldrijan, S. Mutational analysis of Mdm2 C-terminal tail suggests an evolutionarily conserved role of its length in Mdm2 activity toward p53 and indicates structural differences between Mdm2 homodimers and Mdm2/MdmX heterodimers. Cell Cycle 11, 953–962 (2012).
Plechanovová, A. et al. Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat. Struct. Mol. Biol. 18, 1052–1059 (2011).
Honda, R., Tanaka, H. & Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997).
Kubbutat, M.H.G., Ludwig, R.L., Levine, A.J. & Vousden, K.H. Analysis of the degradation function of Mdm2. Cell Growth Differ. 10, 87–92 (1999).
Geyer, R.K., Yu, Z.K. & Maki, C.G. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat. Cell Biol. 2, 569–573 (2000).
Zheng, N., Wang, P., Jeffrey, P.D. & Pavletich, N.P. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102, 533–539 (2000).
Brzovic, P.S. et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc. Natl. Acad. Sci. USA 100, 5646–5651 (2003).
Christensen, D.E., Brzovic, P.S. & Klevit, R.E. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat. Struct. Mol. Biol. 14, 941–948 (2007).
Meulmeester, E. et al. Critical role for a central part of Mdm2 in the ubiquitylation of p53. Mol. Cell. Biol. 23, 4929–4938 (2003).
Argentini, M., Barboule, N. & Wasylyk, B. The contribution of the acidic domain of MDM2 to p53 and MDM2 stability. Oncogene 20, 1267–1275 (2001).
Kawai, H., Wiederschain, D. & Yuan, Z.M. Critical contribution of the MDM2 acidic domain to p53 ubiquitination. Mol. Cell. Biol. 23, 4939–4947 (2003).
Dai, M.S. & Lu, H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 279, 44475–44482 (2004).
Dai, M.S. et al. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell. Biol. 24, 7654–7668 (2004).
Choong, M.L., Yang, H., Lee, M.A. & Lane, D.P. Specific activation of the p53 pathway by low dose actinomycin D: a new route to p53 based cyclotherapy. Cell Cycle 8, 2810–2818 (2009).
Chen, C.S. et al. AKT mediates actinomycin D-induced p53 expression. Oncotarget 5, 693–703 (2014).
Pan, Y. & Chen, J. MDM2 promotes ubiquitination and degradation of MDMX. Mol. Cell. Biol. 23, 5113–5121 (2003).
Kawai, H. et al. DNA damage-induced MDMX degradation is mediated by MDM2. J. Biol. Chem. 278, 45946–45953 (2003).
Linares, L.K., Hengstermann, A., Ciechanover, A., Müller, S. & Scheffner, M. HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc. Natl. Acad. Sci. USA 100, 12009–12014 (2003).
Stad, R. et al. Hdmx stabilizes Mdm2 and p53. J. Biol. Chem. 275, 28039–28044 (2000).
Buetow, L. & Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 17, 626–642 (2016).
Cheng, Q. et al. Regulation of MDM2 E3 ligase activity by phosphorylation after DNA damage. Mol. Cell. Biol. 31, 4951–4963 (2011).
Cheng, Q., Song, T., Chen, L. & Chen, J. Autoactivation of the MDM2 E3 ligase by intramolecular interaction. Mol. Cell. Biol. 34, 2800–2810 (2014).
Roxburgh, P. et al. Small molecules that bind the Mdm2 RING stabilize and activate p53. Carcinogenesis 33, 791–798 (2012).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr 50, 760–763 (1994).
Storoni, L.C., McCoy, A.J. & Read, R.J. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Acknowledgements
We would like to thank L. Buetow for her comments on the manuscript and DLS for access to beamlines I24 beamlines (mx8659) that contributed to the results presented here. This work was supported by Cancer Research UK (C596/A17196) and D.T.H. received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 647849).
Author information
Authors and Affiliations
Contributions
K.N., A.K.H. and K.H.V. designed cell-based experiments. K.N. performed all cell-based experiments and analyzed the data. M.K. and D.T.H. performed crystallization and structural determination. M.K., D.K. and D.T.H. performed protein purification and in vitro biochemical assays. G.J.S. performed and analyzed SPR experiments. K.N., A.K.H., K.H.V. and D.T.H. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
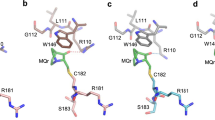
Supplementary Figure 1 UbcH5B~Ub discharge catalyzed by MDM2 and MDM2–MDMX
(a) A representative nonreduced SDS-PAGE showing the effects of UbcH5B mutations in discharging UbcH5B variant~Ub to L-lysine in 1 min catalyzed by MDM2R-MDMXR. (b) A bar graph showing the fraction of UbcH5B variant~Ub left in a. (c) A representative nonreduced SDS-PAGE showing the effects of Ub mutations in discharging UbcH5B~Ub variant to L-lysine in 1 min catalyzed by MDM2R-MDMXR. (d) A bar graph showing the fraction of UbcH5B variant~Ub left in c. (e) A representative nonreduced SDS-PAGE showing the effects of MDM2 mutations in discharging UbcH5B~Ub to L-lysine in 1 min catalyzed by MDM2R-MDMXR variants. (f) A bar graph showing the fraction of UbcH5B~Ub left in e. (g) A representative nonreduced SDS-PAGE showing the discharge of UbcH5B~Ub to L-lysine in 1 min catalyzed by MDM2R-MDMXR variants. (h) A bar graph showing the fraction of UbcH5B~Ub left in g. (i) A representative nonreduced SDS-PAGE showing the discharge of UbcH5B~Ub to L-lysine in 1 min catalyzed by MDM2R-MDMXR variants. (j) A bar graph showing the fraction of UbcH5B~Ub left in i. (k) A representative nonreduced SDS-PAGE showing the discharge of UbcH5B~Ub to L-lysine in 1 min catalyzed by GST-MDM2 variants. (l) A bar graph showing the fraction of UbcH5B~Ub left in k. Three independent reactions were performed (n=3) and error bars indicate standard deviation.
Supplementary Figure 2 Redesigning ligase-dead MDM2 mutants
(a) Nonreduced SDS-PAGE showing the discharge of UbcH5B~Ub to L-lysine over time catalyzed by MDM2 I440 and R479 variants. (b) SDS-PAGE showing the pull-down experiments of GST-MDM2 I440E, I440K and R479P with His-MDM2 I440E, I440K and R479P, respectively. GST-MDM2 variant and the corresponding His-MDM2 variant were co-expressed in E. coli and purified by Ni-NTA affinity followed by glutathione sepharose affinity chromatography. (c) SDS-PAGE showing the pull-down experiments of GST-MDM2 variants with His-MDMX. GST-MDM2 variant and His-MDMX were co-expressed in E. coli and purified by Ni-NTA affinity followed by glutathione sepharose affinity chromatography. (d) Nonreduced SDS-PAGE showing the discharge of UbcH5B~Ub to L-lysine over time catalyzed by MDM2-MDMX variants. Asterisks indicate contaminants.
Supplementary Figure 3 SPR analyses of MDM2 and MDMX variants binding affinities for UbcH5B–Ub
(a) Representative sensorgrams (left) and binding curves (right) for GST-MDM2 398-C variants with UbcH5B–Ub in the presence of UbΔGG are shown. Only sensorgram is shown for GST-MDM2 398-C variants that displayed no measurable UbcH5B–Ub binding in the presence of UbΔGG. (b) Representative sensorgrams (left) and binding curves (right) for GST-MDMXR variants with UbcH5B–Ub are shown. Wild type MDMX displayed no UbcH5B–Ub binding up to 100 μM UbcH5B–Ub whereas both K478R and N448C, K478R mutants exhibited UbcH5B–Ub binding. However, Kd could not be estimated due to the weak binding affinity. All experiments in a and b were performed in duplicates.
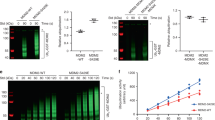
Supplementary Figure 4 Validation of tetracycline-inducible p53 knockdown and MDM2 knockout cells.
U2OS cells were infected with pLKO tet-on shp53. (a) Western blot showing that doxycycline treatment causes p53 knock-down in 2 days and this effect can be washed off in 5 days. (b) MDM2 was then disrupted by CRISPR. Immunoblotting showing CRISPR disruption (target p53 binding domain) resulted in MDM2 knock-out. (c) Genomic PCR followed by sequencing showing that CRISPR disruption (target p53 binding domain) caused MDM2 knock-out. (d) MDM2 knock-out cells were treated with indicated drugs for 4 hours and phosphorylated p53 (serine 15 and serine 20) are analyzed by western blot.
Supplementary Figure 5 MDM2 mutants can limit induction of p53 target genes.
qPCR showing mRNA expression of p53 target genes (GADD45β is not a p53 target gene) is attenuated by MDM2 I440K, I440E but not by C464A. n = 3 independent experiments each. P values (one-way ANOVA with Tukey's post hoc test): v.s. EV No doxy (vertical), v.s. C464A No doxy (horizontal). “n.s.” indicates not significant vs. EV No doxy. F statistics and degrees of freedom of ANOVAs are reported in Supplementary Table 3.
Supplementary Figure 6 MDM2 I440 and R479 mutants can rapidly respond to the stress.
(a) Immunoblots showing that following wild type p53 re-expression by removal of doxycycline (Doxy), p21 is more rapidly induced in cells expressing MDM2 I440 and R479 in response to actinomycin D (10 nM) treatment compared to cells expressing wild type MDM2 (see Fig. 7c). Similar responses in cells treated with 4 μM nutlin (b) and 1 μM doxorubicin (c).
Supplementary Figure 7 Purified proteins used in the assays and size-exclusion chromatography profiles.
(a) SDS-PAGE showing MDM2R-MDMXR variants. (b) SDS-PAGE showing GST-MDM2R-His-MDMXR and GST-MDMXR-His-MDM2R variants. (c) SDS-PAGE showing MDMXR variants, MDM2R-MDMXR and MDMXRH. (d) SDS-PAGE showing GST-MDMXR variants. (e) SDS-PAGE showing GST-MDM2 398-C variants. (f) Superdex 75 10/300 gel filtration chromatography profile of MDM2R-MDMXR and MDMXR variants. Full length MDMX has been reported to dimerize with Kd of 1 μM (Bista, M. et al., Proceedings of the National Academy of Sciences of the United States of America 110, 17814-17819, 2013). To assess the dimerization state of MDMXR variant, protein samples (10 μM, 100 μl) were loaded onto superdex 75 10/300. The dotted line indicates the elution volume of MDM2R-MDMXR heterodimer. MDMXR variants containing N448C substitution eluted similar or slightly earlier than this line indicating dimer formation, whereas MDMX variants lacking N448C substitution eluted later than this line, presumably exist as monomer. (g) HiLoad 26/600 Superdex 200 chromatography profile of MDM2RH purification. His-GST-MDM2R was incubated with TEV protease to release His-GST tag, followed by Ni-NTA pass-back. The cleaved sample was loaded directly onto HiLoad 26/600 Superdex 200. Trace amount of MDM2R was found throughout the elution profile (45-95 ml) with the majority of MDM2R eluted between 95-110 ml consistent with an earlier report showing that MDM2R exists as dimer and aggregate (Poyurovsky, M.V. et al Embo Journal 26, 90-101, 2007). MDM2RH peak (indicated by arrow) eluted after His-GST peak (indicated by arrow; 50 kDa) was assumed as dimer and was pooled and concentrated for activity assay in Fig. 3d.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–6. (PDF 1912 kb)
Supplementary Data Set 1
Uncropped gel and blot images. (PDF 1115 kb)
Rights and permissions
About this article
Cite this article
Nomura, K., Klejnot, M., Kowalczyk, D. et al. Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity. Nat Struct Mol Biol 24, 578–587 (2017). https://doi.org/10.1038/nsmb.3414
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3414
This article is cited by
-
Ubiquitin and a charged loop regulate the ubiquitin E3 ligase activity of Ark2C
Nature Communications (2022)
-
MDMX is essential for the regulation of p53 protein levels in the absence of a functional MDM2 C-terminal tail
BMC Molecular and Cell Biology (2021)
-
Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma
Genome Medicine (2020)
-
Structural basis for DNA damage-induced phosphoregulation of MDM2 RING domain
Nature Communications (2020)
-
Competitive ubiquitination activates the tumor suppressor p53
Cell Death & Differentiation (2020)