Key Points
-
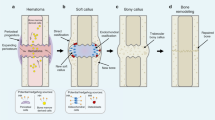
Hedgehog signalling regulates several cellular processes that are critical in bone development
-
Osteoarthritis and cartilaginous neoplasia are associated with activation of the hedgehog signalling pathway
-
Several agents that inhibit hedgehog signalling could be used therapeutically to target this pathway
-
Inhibition of hedgehog signalling or modulation of the expression of downstream target genes are potential modalities for the treatment of osteoarthritis and cartilaginous tumours
-
These two modalities could also be used to increase the effectiveness of strategies to improve bone and joint regeneration
Abstract
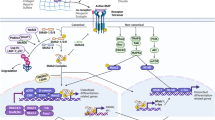
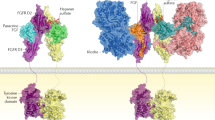
Hedgehog ligands bind to protein patched homologue 1 (PTC), a conserved receptor that activates the GLI family of transcription factors, which are involved in development, disease and skeletal repair processes. During embryonic development, hedgehog signalling helps to pattern the limbs and plays a critical part in regulating chondrocyte differentiation and osteogenesis during the longitudinal growth of long bones. This signalling pathway also regulates mesenchymal cell differentiation during skeletal repair and regeneration. In pathologic and degenerative processes, such as osteoarthritis or cartilaginous tumour formation, hedgehog signalling is dysregulated. Several pharmacologic strategies can modulate hedgehog signalling, and targeting this pathway could lead to the development of novel therapeutic approaches. For example, by precisely regulating the level of activity of the hedgehog signalling pathway, the pace of degeneration in osteoarthritis could be slowed, bone repair could be enhanced, and cartilaginous tumour cell viability could be inhibited. As such, regulation of hedgehog signalling could be manipulated to safeguard skeletal health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gofflot, F. et al. Molecular mechanisms underlying limb anomalies associated with cholesterol deficiency during gestation: implications of hedgehog signaling. Hum. Mol. Genet. 12, 1187–1198 (2003).
Varjosalo, M. & Taipale, J. Hedgehog: functions and mechanisms. Genes Dev. 22, 2454–2472 (2008).
Jiang, J. & Hui, C. C. Hedgehog signaling in development and cancer. Dev. Cell 15, 801–812 (2008).
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Turnpenny, P. D. et al. Abnormal vertebral segmentation and the notch signaling pathway in man. Dev. Dyn. 236, 1456–1474 (2007).
Tiet, T. D. & Alman, B. A. Developmental pathways in musculoskeletal neoplasia: involvement of the Indian Hedgehog–parathyroid hormone-related protein pathway. Pediatr. Res. 53, 539–543 (2003).
Spitz, F. & Duboule, D. Development. The art of making a joint. Science 291, 1713–1714 (2001).
Khillan, J. S. Generation of chondrocytes from embryonic stem cells. Methods Mol. Biol. 330, 161–170 (2006).
Kramer, J., Hegert, C., Hargus, G. & Rohwedel, J. Chondrocytes derived from mouse embryonic stem cells. Cytotechnology 41, 177–187 (2003).
Tuli, R. et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells 21, 681–693 (2003).
Prockop, D. J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 17, 939–946 (2009).
Noth, U. et al. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J. Orthop. Res. 20, 1060–1069 (2002).
Hwang, N. S., Varghese, S. & Elisseeff, J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE 3, e2498 (2008).
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Yang, L., Tsang, K. Y., Tang, H. C., Chan, D. & Cheah, K. S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl Acad. Sci. USA 111, 12097–12102 (2014).
Pacifici, M. et al. Hypertrophic chondrocytes. The terminal stage of differentiation in the chondrogenic cell lineage? Ann. NY Acad. Sci. 599, 45–57 (1990).
Chevy, F., Illien, F., Wolf, C. & Roux, C. Limb malformations of rat fetuses exposed to a distal inhibitor of cholesterol biosynthesis. J. Lipid Res. 43, 1192–1200 (2002).
Roux, C. et al. Role of cholesterol in embryonic development. Am. J. Clin. Nutr. 71 (Suppl.), 1270S–1279S (2000).
Cortes, M., Baria, A. T. & Schwartz, N. B. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development 136, 1697–1706 (2009).
Guerrero, I. & Chiang, C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol. 17, 1–5 (2007).
Varjosalo, M., Li, S. P. & Taipale, J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev. Cell 10, 177–186 (2006).
Liu, Y., Cao, X., Jiang, J. & Jia, J. Fused–Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes Dev. 21, 1949–1963 (2007).
Chen, C. H. et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell 98, 305–316 (1999).
Murone, M. et al. Gli regulation by the opposing activities of Fused and Suppressor of fused. Nat. Cell Biol. 2, 310–312 (2000).
Pan, Y. & Wang, B. A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. J. Biol. Chem. 282, 10846–10852 (2007).
Marigo, V. & Tabin, C. J. Regulation of Patched by Sonic hedgehog in the developing neural tube. Proc. Natl Acad. Sci. USA 93, 9346–9351 (1996).
Hojo, H. et al. Gli1 protein participates in hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J. Biol. Chem. 287, 17860–17869 (2012).
Kesper, D. A., Didt-Koziel, L. & Vortkamp, A. Gli2 activator function in preosteoblasts is sufficient to mediate Ihh-dependent osteoblast differentiation, whereas the repressor function of Gli2 is dispensable for endochondral ossification. Dev. Dyn. 239, 1818–1826 (2010).
Johnson, R. L., Grenier, J. K. & Scott, M. P. Patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development 121, 4161–4170 (1995).
Holtz, A. M. et al. Essential role for ligand-dependent feedback antagonism of vertebrate hedgehog signaling by PTCH1, PTCH2 and HHIP1 during neural patterning. Development 140, 3423–3434 (2013).
Chuang, P. T., Kawcak, T. & McMahon, A. P. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 17, 342–347 (2003).
Kogerman, P. et al. Mammalian suppressor-of-fused modulates nuclear–cytoplasmic shuttling of Gli-1. Nat. Cell Biol. 1, 312–319 (1999).
Katoh, Y. & Katoh, M. KIF27 is one of orthologs for Drosophila Costal-2. Int. J. Oncol. 25, 1875–1880 (2004).
Dunaeva, M., Michelson, P., Kogerman, P. & Toftgard, R. Characterization of the physical interaction of Gli proteins with SUFU proteins. J. Biol. Chem. 278, 5116–5122 (2003).
Katoh, Y. & Katoh, M. Characterization of KIF7 gene in silico. Int. J. Oncol. 25, 1881–1886 (2004).
Wheatley, D. N. Primary cilia in normal and pathological tissues. Pathobiology 63, 222–238 (1995).
Liu, A., Wang, B. & Niswander, L. A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Song, B., Haycraft, C. J., Seo, H. S., Yoder, B. K. & Serra, R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 305, 202–216 (2007).
Poole, C. A., Zhang, Z. J. & Ross, J. M. The differential distribution of acetylated and detyrosinated α-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J. Anat. 199, 393–405 (2001).
Huangfu, D. & Anderson, K. V. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14 (2006).
Haycraft, C. J. et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, e53 (2005).
Malone, A. M. et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl Acad. Sci. USA 104, 13325–13330 (2007).
Vortkamp, A. et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613–622 (1996).
Karp, S. J. et al. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127, 543–548 (2000).
Kobayashi, T. et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129, 2977–2986 (2002).
Lanske, B. et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666 (1996).
Minina, E. et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128, 4523–4534 (2001).
Minina, E., Kreschel, C., Naski, M. C., Ornitz, D. M. & Vortkamp, A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3, 439–449 (2002).
Koziel, L., Wuelling, M., Schneider, S. & Vortkamp, A. Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development 132, 5249–5260 (2005).
Miao, D. et al. Impaired endochondral bone development and osteopenia in Gli2-deficient mice. Exp. Cell Res. 294, 210–222 (2004).
Mau, E. et al. PTHrP regulates growth plate chondrocyte differentiation and proliferation in a Gli3 dependent manner utilizing hedgehog ligand dependent and independent mechanisms. Dev. Biol. 305, 28–39 (2007).
Epstein, D. J., Marti, E., Scott, M. P. & McMahon, A. P. Antagonizing cAMP-dependent protein kinase A in the dorsal CNS activates a conserved sonic hedgehog signaling pathway. Development 122, 2885–2894 (1996).
Riobo, N. A., Lu, K., Ai, X., Haines, G. M. & Emerson, C. P. Jr. Phosphoinositide 3-kinase and Akt are essential for sonic hedgehog signaling. Proc. Natl Acad. Sci. USA 103, 4505–4510 (2006).
Yang, Y., Guillot, P., Boyd, Y., Lyon, M. F. & McMahon, A. P. Evidence that preaxial polydactyly in the Doublefoot mutant is due to ectopic Indian hedgehog signaling. Development 125, 3123–3132 (1998).
Radhakrishna, U., Wild, A., Grzeschik, K. H. & Antonarakis, S. E. Mutation in GLI3 in postaxial polydactyly type A. Nat. Genet. 17, 269–271 (1997).
Hui, C. C. & Joyner, A. L. A mouse model of Greig cephalopolysyndactyly syndrome: the extra-toes J mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 3, 241–246 (1993).
Gao, B. et al. Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat. Genet. 28, 386–388 (2001).
Spjut, H. J., Dorfman, H. D., Fechner, R. E. & Ackerman, L. V. (eds) Tumours of Bone and Cartilage, Atlas of Tumor Pathology, Second Series, Fasicle 5. 77–110 (Armed Forces Institute of Pathology, 1983).
Levesque, J. et al. (eds) A Clinical Guide to Primary Bone Tumors 98–107 (Williams and Wilkins, 1998).
Schwartz, H. S. et al. The malignant potential of enchondromatosis. J. Bone Joint Surg. Am. 69, 269–274 (1987).
Wuyts, W. & Van Hul, W. Molecular basis of multiple exostoses: mutations in the EXT1 and EXT2 genes. Hum. Mutat. 15, 220–227 (2000).
McCormick, C. et al. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat. Genet. 19, 158–161 (1998).
Hecht, J. T. et al. Differentiation-induced loss of heparan sulfate in human exostosis derived chondrocytes. Differentiation 73, 212–221 (2005).
Alvarez, C., Tredwell, S., De Vera, M. & Hayden, M. The genotype–phenotype correlation of hereditary multiple exostoses. Clin. Genet. 70, 122–130 (2006).
Hopyan, S. et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nat. Genet. 30, 306–310 (2002).
Couvineau, A. et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum. Mol. Genet. 17, 2766–2775 (2008).
Ho, L. et al. Gli2 and p53 cooperate to regulate IGFBP-3-mediated chondrocyte apoptosis in the progression from benign to malignant cartilage tumors. Cancer Cell. 16, 126–136 (2009).
Huang, K. & Wu, L. D. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J. Int. Med. Res. 36, 1149–1160 (2008).
Bondeson, J., Wainwright, S., Hughes, C. & Caterson, B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin. Exp. Rheumatol. 26, 139–145 (2008).
Appleton, C. T., Pitelka, V., Henry, J. & Beier, F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 56, 1854–1868 (2007).
Tchetina, E. V., Squires, G. & Poole, A. R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 32, 876–886 (2005).
Aigner, T., Soder, S., Gebhard, P. M., McAlinden, A. & Haag, J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis—structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 3, 391–399 (2007).
Mak, K. K., Kronenberg, H. M., Chuang, P. T., Mackem, S. & Yang, Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 135, 1947–1956 (2008).
Lin, A. C. et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat. Med. 15, 1421–1425 (2009).
Ruiz-Heiland, G. et al. Blockade of the hedgehog pathway inhibits osteophyte formation in arthritis. Ann. Rheum. Dis. 71, 400–407 (2012).
Zhou, J. et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2 Ihhfl/fl mice. Arthritis Res. Ther. 16, R11 (2014).
Chang, C. F., Ramaswamy, G. & Serra, R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage 20, 152–161 (2012).
Regard, J. B. et al. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat. Med. 19, 1505–1512 (2013).
Buckland, J. Bone: Hedgehog signalling linked to heterotopic ossification in POH. Nat. Rev. Rheumatol. 9, 636 (2013).
Schmidts, M. et al. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am. J. Hum. Genet. 93, 932–944 (2013).
Yang, S. & Wang, C. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone 51, 407–417 (2012).
Ohba, S. et al. Patched1 haploinsufficiency increases adult bone mass and modulates Gli3 repressor activity. Dev. Cell. 14, 689–699 (2008).
Vortkamp, A. et al. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech. Dev. 71, 65–76 (1998).
Murakami, S. & Noda, M. Expression of Indian hedgehog during fracture healing in adult rat femora. Calcif. Tissue Int. 66, 272–276 (2000).
Baht, G. S., Silkstone, D., Nadesan, P., Whetstone, H. & Alman, B. A. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J. Orthop. Res. 32, 581–586 (2014).
Craft, A. M. et al. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 140, 2597–2610 (2013).
Rubin, L. L. & de Sauvage, F. J. Targeting the hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026–1033 (2006).
De Smaele, E., Ferretti, E. & Gulino, A. Vismodegib, a small-molecule inhibitor of the hedgehog pathway for the treatment of advanced cancers. Curr. Opin. Investig. Drugs 11, 707–718 (2010).
Rockel, J. S. & Alman, B. A. Don't hedge your bets: hedgehog signaling as a central mediator of endochondral bone development and cartilage diseases. J. Orthop. Res. 29, 810–815 (2011).
Kimura, H., Ng, J. M. & Curran, T. Transient inhibition of the hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell 13, 249–260 (2008).
Kim, J. et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell 17, 388–399 (2010).
Aravamudhan, A. et al. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr. Pharm. Des 19, 3420–3428 (2013).
Gellynck, K. et al. Small molecule stimulation enhances bone regeneration but not titanium implant osseointegration. Bone 57, 405–412 (2013).
Acknowledgements
The author's research referenced in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH under Award R01AR066765-01 to B.A.A. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Alman, B. The role of hedgehog signalling in skeletal health and disease. Nat Rev Rheumatol 11, 552–560 (2015). https://doi.org/10.1038/nrrheum.2015.84
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.84
This article is cited by
-
Prenatal diagnosis to identify compound heterozygous variants in PKDCC that causes rhizomelic limb shortening with dysmorphic features in a fetus from China
BMC Medical Genomics (2023)
-
Hedgehog signaling regulates bone homeostasis through orchestrating osteoclast differentiation and osteoclast–osteoblast coupling
Cellular and Molecular Life Sciences (2023)
-
N-myc downstream regulated gene 1 suppresses osteoblast differentiation through inactivating Wnt/β-catenin signaling
Stem Cell Research & Therapy (2022)
-
GSK-3β suppression upregulates Gli1 to alleviate osteogenesis inhibition in titanium nanoparticle-induced osteolysis
Journal of Nanobiotechnology (2022)
-
Metastasis suppressor 1 controls osteoblast differentiation and bone homeostasis through regulating Src-Wnt/β-catenin signaling
Cellular and Molecular Life Sciences (2022)