Key Points
-
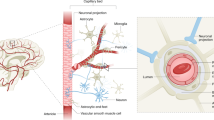
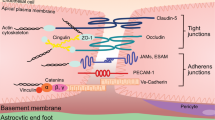
The blood–brain barrier (BBB) protects neurons from factors present in the systemic circulation and maintains the highly regulated brain internal milieu, which is required for proper synaptic and neuronal functioning
-
BBB breakdown facilitates entry into the brain of neurotoxic blood-derived products, cells and pathogens and is associated with inflammatory and immune responses, which can initiate multiple neurodegenerative pathways
-
Neuroimaging studies have demonstrated early BBB dysfunction in Alzheimer disease and other neurodegenerative disorders, which is also supported by biofluid biomarker data and is consistently observed in post-mortem tissue
-
BBB dysfunction in neurodegenerative disorders includes increased BBB permeability, microbleeds, impaired glucose transport, impaired P-glycoprotein 1 function, perivascular deposits of blood-derived products, cellular infiltration and degeneration of pericytes and endothelial cells
Abstract
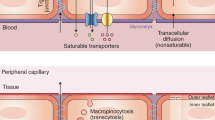
The blood–brain barrier (BBB) is a continuous endothelial membrane within brain microvessels that has sealed cell-to-cell contacts and is sheathed by mural vascular cells and perivascular astrocyte end-feet. The BBB protects neurons from factors present in the systemic circulation and maintains the highly regulated CNS internal milieu, which is required for proper synaptic and neuronal functioning. BBB disruption allows influx into the brain of neurotoxic blood-derived debris, cells and microbial pathogens and is associated with inflammatory and immune responses, which can initiate multiple pathways of neurodegeneration. This Review discusses neuroimaging studies in the living human brain and post-mortem tissue as well as biomarker studies demonstrating BBB breakdown in Alzheimer disease, Parkinson disease, Huntington disease, amyotrophic lateral sclerosis, multiple sclerosis, HIV-1-associated dementia and chronic traumatic encephalopathy. The pathogenic mechanisms by which BBB breakdown leads to neuronal injury, synaptic dysfunction, loss of neuronal connectivity and neurodegeneration are described. The importance of a healthy BBB for therapeutic drug delivery and the adverse effects of disease-initiated, pathological BBB breakdown in relation to brain delivery of neuropharmaceuticals are briefly discussed. Finally, future directions, gaps in the field and opportunities to control the course of neurological diseases by targeting the BBB are presented.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zlokovic, B. V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Kisler, K., Nelson, A. R., Montagne, A. & Zlokovic, B. V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
Iadecola, C. The pathobiology of vascular dementia. Neuron 80, 844–866 (2013).
Pardridge, W. M. Targeted delivery of protein and gene medicines through the blood–brain barrier. Clin. Pharmacol. Ther. 97, 347–361 (2015).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood–brain barrier. Cell 163, 1064–1078 (2015).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Mann, G. E., Zlokovic, B. V. & Yudilevich, D. L. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim. Biophys. Acta 819, 241–248 (1985).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Montagne, A., Zhao, Z. & Zlokovic, B. Alzheimer's disease: a matter of blood–brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Sakadžic, S. et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat. Commun. 5, 5734 (2014).
Kisler, K. et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416 (2017).
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509, 507–511 (2014).
Mokgokong, R., Wang, S., Taylor, C. J., Barrand, M. A. & Hladky, S. B. Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch. 466, 887–901 (2014).
Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R. & Begley, D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 37, 13–25 (2010).
Vazana, U. et al. Glutamate-mediated blood–brain barrier opening: implications for neuroprotection and drug delivery. J. Neurosci. 36, 7727–7739 (2016).
Shibata, M. et al. Clearance of Alzheimer's amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J. Clin. Invest. 106, 1489–1499 (2000).
Deane, R. et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43, 333–344 (2004).
Bell, R. D. et al. Transport pathways for clearance of human Alzheimer's amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J. Cereb. Blood Flow Metab. 27, 909–918 (2007).
Deane, R. et al. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013 (2008).
Zlokovic, B. V. Neurodegeneration and the neurovascular unit. Nat. Med. 16, 1370–1371 (2010).
Storck, S. E. et al. Endothelial LRP1 transports amyloid-β1–42 across the blood–brain barrier. J. Clin. Invest. 126, 123–136 (2016).
Zhao, Z. et al. Central role for PICALM in amyloid-β blood–brain barrier transcytosis and clearance. Nat. Neurosci. 18, 978–987 (2015).
Saito, S. & Ihara, M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr. Opin. Psychiatry 29, 168–173 (2016).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain — implications for Alzheimer disease. Nat. Rev. Neurol. 12, 248 (2016).
Bakker, E. N. et al. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 36, 181–194 (2016).
Bradbury, M. W., Cserr, H. F. & Westrop, R. J. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 240, F329–F336 (1981).
Ichimura, T., Fraser, P. A. & Cserr, H. F. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 545, 103–113 (1991).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Engelhardt, B. et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 132, 317–338 (2016).
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131 (2017).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Rennels, M. L., Gregory, T. F., Blaumanis, O. R., Fujimoto, K. & Grady, P. A. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326, 47–63 (1985).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111 (2012).
Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: a beginner's guide. Neurochem. Res. 40, 2583–2599 (2015).
Smith, A. J., Yao, X., Dix, J. A., Jin, B.-J. & Verkman, A. S. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6, e27679 (2017).
Holter, K. E. et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl Acad. Sci. USA 114, 9894–9899 (2017).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 (2014).
Spector, R., Robert Snodgrass, S. & Johanson, C. E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 273, 57–68 (2015).
Asgari, N., Berg, C. T., Mørch, M. T., Khorooshi, R. & Owens, T. Cerebrospinal fluid aquaporin-4-immunoglobulin G disrupts blood brain barrier. Ann. Clin. Transl Neurol. 2, 857–863 (2015).
Asgari, M., de Zélicourt, D. & Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Sci. Rep. 6, 38635 (2016).
Jin, B.-J., Smith, A. J. & Verkman, A. S. Spatial model of convective solute transport in brain extracellular space does not support a 'glymphatic' mechanism. J. Gen. Physiol. 148, 489–501 (2016).
Montagne, A. et al. Brain imaging of neurovascular dysfunction in Alzheimer's disease. Acta Neuropathol. 131, 687–707 (2016).
Nelson, A. R., Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochim. Biophys. Acta 1862, 887–900 (2016).
Iturria-Medina, Y. et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer's disease. J. Cereb. Blood Flow Metab. 35, 1055–1068 (2015).
Arvanitakis, Z., Capuano, A. W., Leurgans, S. E., Bennett, D. A. & Schneider, J. A. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943 (2016).
Toledo, J. B. et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain J. Neurol. 136, 2697–2706 (2013).
Rosenberg, G. A. Blood–brain barrier permeability in aging and Alzheimer's disease. J. Prev. Alzheimers Dis. 1, 138–139 (2014).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 12, 383–388 (1991).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Montine, T. J. et al. Recommendations of the Alzheimer's Disease-Related Dementias Conference. Neurology 83, 851–860 (2014).
Snyder, H. M. et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. J. 11, 710–717 (2015).
Hachinski, V. & World Stroke Organization. Stroke and potentially preventable dementias proclamation: updated World Stroke Day proclamation. Stroke 46, 3039–3040 (2015).
Malek, N. et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov. Disord. 31, 1518–1526 (2016).
Drouin-Ouellet, J. et al. Cerebrovascular and blood–brain barrier impairments in Huntington's disease: potential implications for its pathophysiology. Ann. Neurol. 78, 160–177 (2015).
Lin, C.-Y. et al. Neurovascular abnormalities in humans and mice with Huntington's disease. Exp. Neurol. 250, 20–30 (2013).
Winkler, E. A. et al. Blood–spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 125, 111–120 (2013).
Doherty, C. P. et al. Blood–brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 75, 656–662 (2016).
Strazza, M., Pirrone, V., Wigdahl, B. & Nonnemacher, M. R. Breaking down the barrier: the effects of HIV-1 on the blood–brain barrier. Brain Res. 1399, 96–115 (2011).
Barnes, S. R. et al. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med. Imag. 15, 19 (2015).
Barnes, S. R. et al. Optimal acquisition and modeling parameters for accurate assessment of low K trans blood–brain barrier permeability using dynamic contrast-enhanced MRI. Magn. Reson. Med. 75, 1967–1977 (2016).
Sagare, A. P., Sweeney, M. D., Makshanoff, J. & Zlokovic, B. V. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci. Lett. 607, 97–101 (2015).
Whitwell, J. L. et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case–control study. Lancet Neurol. 11, 868–877 (2012).
Apostolova, L. G. et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol. Aging 31, 1077–1088 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Winkler, E. A., Sengillo, J. D., Bell, R. D., Wang, J. & Zlokovic, B. V. Blood–spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 32, 1841–1852 (2012).
Winkler, E. A. et al. Blood–spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl Acad. Sci. USA 111, E1035–E1042 (2014).
van de Haar, H. J. et al. Blood–brain barrier leakage in patients with early Alzheimer disease. Radiology 281, 527–535 (2016).
van de Haar, H. J. et al. Neurovascular unit impairment in early Alzheimer's disease measured with magnetic resonance imaging. Neurobiol. Aging 45, 190–196 (2016).
van de Haar, H. J. et al. Subtle blood–brain barrier leakage rate and spatial extent: considerations for dynamic contrast-enhanced MRI. Med. Phys. 44, 4112–4125 (2017).
Wang, H., Golob, E. J. & Su, M.-Y. Vascular volume and blood–brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J. Magn. Reson. Imag. 24, 695–700 (2006).
Starr, J. M., Farrall, A. J., Armitage, P., McGurn, B. & Wardlaw, J. Blood–brain barrier permeability in Alzheimer's disease: a case–control MRI study. Psychiatry Res. 171, 232–241 (2009).
Al-Bachari, S. MRI assessment of neurovascular changes in idiopathic Parkinson's disease. Thesis, Univ. Manchester (2016).
Taheri, S., Gasparovic, C., Shah, N. J. & Rosenberg, G. A. Quantitative measurement of blood–brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn. Reson. Med. 65, 1036–1042 (2011).
Cramer, S. P., Simonsen, H., Frederiksen, J. L., Rostrup, E. & Larsson, H. B. W. Abnormal blood–brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 4, 182–189 (2014).
Cramer, S. P., Modvig, S., Simonsen, H. J., Frederiksen, J. L. & Larsson, H. B. W. Permeability of the blood–brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain J. Neurol. 138, 2571–2583 (2015).
Gaitán, M. I. et al. Evolution of the blood–brain barrier in newly forming multiple sclerosis lesions. Ann. Neurol. 70, 22–29 (2011).
Ingrisch, M. et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest. Radiol. 47, 252–258 (2012).
Fainardi, E. et al. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult. Scler. 12, 294–301 (2006).
Goos, J. D. C. et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40, 3455–3460 (2009).
Brundel, M. et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer's disease. J. Alzheimers Dis. 31, 259–263 (2012).
Uetani, H. et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am. J. Neuroradiol. 34, 984–989 (2013).
Zonneveld, H. I. et al. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology 82, 698–704 (2014).
Olazarán, J. et al. Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am. J. Alzheimers Dis. Other Demen. 29, 263–269 (2014).
Heringa, S. M. et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer's disease. J. Alzheimers Dis. 38, 211–221 (2014).
Shams, S. et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis — the Karolinska Imaging Dementia Study. AJNR Am. J. Neuroradiol. 36, 661–666 (2015).
Poliakova, T., Levin, O., Arablinskiy, A., Vasenina, E. & Zerr, I. Cerebral microbleeds in early Alzheimer's disease. J. Neurol. 263, 1961–1968 (2016).
Yates, P. A. et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82, 1266–1273 (2014).
Greenberg, S. M. et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 8, 165–174 (2009).
Viswanathan, A. & Greenberg, S. M. Cerebral amyloid angiopathy in the elderly. Ann. Neurol. 70, 871–880 (2011).
Hanyu, H., Tanaka, Y., Shimizu, S., Takasaki, M. & Abe, K. Cerebral microbleeds in Alzheimer's disease. J. Neurol. 250, 1496–1497 (2003).
Pettersen, J. A. et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch. Neurol. 65, 790–795 (2008).
Kantarci, K. et al. Focal hemosiderin deposits and β-amyloid load in the ADNI cohort. Alzheimers Dement. 9, S116–S123 (2013).
Feldman, H. H. et al. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke 39, 2894–2897 (2008).
Charidimou, A. et al. Cortical superficial siderosis in memory clinic patients: further evidence for underlying cerebral amyloid angiopathy. Cerebrovasc. Dis. 41, 156–162 (2016).
Shams, S. et al. Cortical superficial siderosis: prevalence and biomarker profile in a memory clinic population. Neurology 87, 1110–1117 (2016).
Blair, G. W., Hernandez, M. V., Thrippleton, M. J., Doubal, F. N. & Wardlaw, J. M. Advanced neuroimaging of cerebral small vessel disease. Curr. Treat. Opt. Cardiovasc. Med. 19, 56 (2017).
Shams, S. & Wahlund, L.-O. Cerebral microbleeds as a biomarker in Alzheimer's disease? A review in the field. Biomark. Med. 10, 9–18 (2016).
Ham, J. H. et al. Cerebral microbleeds in patients with Parkinson's disease. J. Neurol. 261, 1628–1635 (2014).
Janelidze, S. et al. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology 85, 1834–1842 (2015).
Kwan, J. Y. et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 Tesla MRI and pathology. PLoS ONE 7, e35241 (2012).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18, 521–530 (2015).
Sokoloff, L. et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916 (1977).
McDougal, D. B. et al. Use of nonradioactive 2-deoxyglucose to study compartmentation of brain glucose metabolism and rapid regional changes in rate. Proc. Natl Acad. Sci. USA 87, 1357–1361 (1990).
Cunnane, S. et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 27, 3–20 (2011).
Crane, R. K. & Sols, A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J. Biol. Chem. 210, 597–606 (1954).
Rokka, J., Grönroos, T. J., Viljanen, T., Solin, O. & Haaparanta-Solin, M. HPLC and TLC methods for analysis of [18F]FDG and its metabolites from biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 1048, 140–149 (2017).
Southworth, R., Parry, C. R., Parkes, H. G., Medina, R. A. & Garlick, P. B. Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR spectroscopy study in the rat. NMR Biomed. 16, 494–502 (2003).
Hers, H. G. & De Duve, C. The hexosephosphatase system; partition of activity of glucose-6-phosphatase in the tissues [French]. Bull. Soc. Chim. Biol. (Paris) 32, 20–29 (1950).
Sokoloff, L. Measurement of local cerebral glucose utilization and its relation to local functional activity in the brain. Adv. Exp. Med. Biol. 291, 21–42 (1991).
Huang, M. T. & Veech, R. L. Metabolic fluxes between [14C]2-deoxy-D-glucose and [14C]2-deoxy-D-glucose-6-phosphate in brain in vivo. J. Neurochem. 44, 567–573 (1985).
Simpson, I. A., Chundu, K. R., Davies-Hill, T., Honer, W. G. & Davies, P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann. Neurol. 35, 546–551 (1994).
Mooradian, A. D., Chung, H. C. & Shah, G. N. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol. Aging 18, 469–474 (1997).
Kalaria, R. N. & Harik, S. I. Reduced glucose transporter at the blood–brain barrier and in cerebral cortex in Alzheimer disease. J. Neurochem. 53, 1083–1088 (1989).
Horwood, N. & Davies, D. C. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer's disease. Virchows Arch. 425, 69–72 (1994).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 280–292 (2011).
Hunt, A. et al. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Res. 155, 147–154 (2007).
Samuraki, M. et al. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imag. 34, 1658–1669 (2007).
Mosconi, L. et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J. Nucl. Med. 49, 390–398 (2008).
Mosconi, L. et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J. Nucl. Med. 47, 1778–1786 (2006).
Landau, S. M. et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 32, 1207–1218 (2011).
Bailly, M. et al. Precuneus and cingulate cortex atrophy and hypometabolism in patients with Alzheimer's disease and mild cognitive impairment: MRI and 18F-FDG PET quantitative analysis using FreeSurfer. Biomed. Res. Int. 2015, 583931 (2015).
Ossenkoppele, R. et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 80, 359–365 (2013).
Protas, H. D. et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 70, 320–325 (2013).
Mosconi, L. et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer's parents. Neurobiol. Aging 34, 22–34 (2013).
Landau, S. M. et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–238 (2010).
Mosconi, L. et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imag. 36, 811–822 (2009).
Niwa, K., Kazama, K., Younkin, S. G., Carlson, G. A. & Iadecola, C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 9, 61–68 (2002).
Jagust, W. J. et al. Diminished glucose transport in Alzheimer's disease: dynamic PET studies. J. Cereb. Blood Flow Metab. 11, 323–330 (1991).
Piert, M., Koeppe, R. A., Giordani, B., Berent, S. & Kuhl, D. E. Diminished glucose transport and phosphorylation in Alzheimer's disease determined by dynamic FDG-PET. J. Nucl. Med. 37, 201–208 (1996).
Cirrito, J. R. et al. P-Glycoprotein deficiency at the blood–brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290 (2005).
Wang, W., Bodles-Brakhop, A. M. & Barger, S. W. A role for P-glycoprotein in clearance of Alzheimer amyloid β -peptide from the brain. Curr. Alzheimer Res. 13, 615–620 (2016).
McInerney, M. P., Short, J. L. & Nicolazzo, J. A. Neurovascular alterations in Alzheimer's disease: transporter expression profiles and CNS drug access. AAPS J. 19, 940–956 (2017).
van Assema, D. M. E. et al. Blood–brain barrier P-glycoprotein function in Alzheimer's disease. Brain J. Neurol. 135, 181–189 (2012).
Deo, A. K. et al. Activity of P-glycoprotein, a β-amyloid transporter at the blood–brain barrier, is compromised in patients with mild Alzheimer disease. J. Nucl. Med. 55, 1106–1111 (2014).
Kortekaas, R. et al. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol 57, 176–179 (2005).
Gerwien, H. et al. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood–brain barrier. Sci. Transl Med. 8, 364ra152 (2016).
Zamboni, P. et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J. Neurol. Sci. 282, 21–27 (2009).
Marshall, O. et al. Impaired cerebrovascular reactivity in multiple sclerosis. JAMA Neurol. 71, 1275–1281 (2014).
Cullen, K. M., Kócsi, Z. & Stone, J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J. Cereb. Blood Flow Metab. 25, 1656–1667 (2005).
Hultman, K., Strickland, S. & Norris, E. H. The APOEε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J. Cereb. Blood Flow Metab. 33, 1251–1258 (2013).
Zenaro, E. et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Fiala, M. et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood–brain barrier. Eur. J. Clin. Invest. 32, 360–371 (2002).
Persidsky, Y. et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood–brain barrier in HIV-1 encephalitis (HIVE). Blood 107, 4770–4780 (2006).
Omalu, B. I., Fitzsimmons, R. P., Hammers, J. & Bailes, J. Chronic traumatic encephalopathy in a professional American wrestler. J. Forens. Nurs. 6, 130–136 (2010).
Zipser, B. D. et al. Microvascular injury and blood–brain barrier leakage in Alzheimer's disease. Neurobiol. Aging 28, 977–986 (2007).
Ryu, J. K. & McLarnon, J. G. A leaky blood–brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J. Cell. Mol. Med. 13, 2911–2925 (2009).
Cortes-Canteli, M. et al. Fibrinogen and β-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron 66, 695–709 (2010).
Sengillo, J. D. et al. Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer's disease. Brain Pathol. 23, 303–310 (2013).
Halliday, M. R. et al. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J. Cereb. Blood Flow Metab. 36, 216–227 (2016).
Miners, J. S., Schulz, I. & Love, S. Differing associations between Aβ accumulation, hypoperfusion, blood–brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer's disease. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/0271678X17690761 (2017).
Salloway, S. et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer's disease. J. Neurol. Sci. 203–204, 183–187 (2002).
Sagare, A. P. et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 4, 2932 (2013).
Park, L. et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 110, 3089–3094 (2013).
Kelly, P. et al. Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic mice following treatment with Liraglutide™. Microcirculation 22, 133–145 (2015).
Park, J.-C. et al. Annexin A1 restores Aβ1–42-induced blood–brain barrier disruption through the inhibition of RhoA–ROCK signaling pathway. Aging Cell 16, 149–161 (2017).
Alata, W., Ye, Y., St-Amour, I., Vandal, M. & Calon, F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood–brain barrier function in mice. J. Cereb. Blood Flow Metab. 35, 86–94 (2015).
Nishitsuji, K., Hosono, T., Nakamura, T., Bu, G. & Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood–brain barrier model. J. Biol. Chem. 286, 17536–17542 (2011).
Gray, M. T. & Woulfe, J. M. Striatal blood–brain barrier permeability in Parkinson's disease. J. Cereb. Blood Flow Metab. 35, 747–750 (2015).
Pienaar, I. S. et al. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson's disease. Neurobiol. Dis. 74, 392–405 (2015).
Loeffler, D. A. et al. Transferrin and iron in normal, Alzheimer's disease, and Parkinson's disease brain regions. J. Neurochem. 65, 710–724 (1995).
Garbuzova-Davis, S. et al. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 1469, 114–128 (2012).
Zhong, Z. et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 11, 420–422 (2008).
Kirk, J., Plumb, J., Mirakhur, M. & McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 201, 319–327 (2003).
Omalu, B. I. et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128–134 (2005).
Farkas, E. & Luiten, P. G. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 64, 575–611 (2001).
Baloyannis, S. J. & Baloyannis, I. S. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J. Neurol. Sci. 322, 117–121 (2012).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Niu, F., Yao, H., Zhang, W., Sutliff, R. L. & Buch, S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J. Neurosci. 34, 11812–11825 (2014).
Kokjohn, T. A. et al. Neurochemical profile of dementia pugilistica. J. Neurotrauma 30, 981–997 (2013).
Bailey, T. L., Rivara, C. B., Rocher, A. B. & Hof, P. R. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol. Res. 26, 573–578 (2004).
Wu, Z. et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 11, 959–965 (2005).
Grammas, P., Tripathy, D., Sanchez, A., Yin, X. & Luo, J. Brain microvasculature and hypoxia-related proteins in Alzheimer's disease. Int. J. Clin. Exp. Pathol. 4, 616–627 (2011).
Henkel, J. S., Beers, D. R., Wen, S., Bowser, R. & Appel, S. H. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 72, 1614–1616 (2009).
Miyazaki, K. et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res. 89, 718–728 (2011).
Yamamoto, M. et al. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 172, 521–533 (2008).
Kumar, D. K. V. et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci. Transl Med. 8, 340ra72 (2016).
Soscia, S. J. et al. The Alzheimer's disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE 5, e9505 (2010).
Wada, K. et al. Expression levels of vascular endothelial growth factor and its receptors in Parkinson's disease. Neuroreport 17, 705–709 (2006).
Desai Bradaric, B., Patel, A., Schneider, J. A., Carvey, P. M. & Hendey, B. Evidence for angiogenesis in Parkinson's disease, incidental Lewy body disease, and progressive supranuclear palsy. J. Neural Transm. 119, 59–71 (2012).
Hill, K. K. et al. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson's disease. Exp. Neurol. 241, 105–112 (2013).
Donahue, J. E. et al. RAGE, LRP-1, and amyloid-β protein in Alzheimer's disease. Acta Neuropathol. 112, 405–415 (2006).
Miller, M. C. et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 1230, 273–280 (2008).
Sagare, A. P., Deane, R. & Zlokovic, B. V. Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharmacol. Ther. 136, 94–105 (2012).
DeMattos, R. B., Bales, K. R., Cummins, D. J., Paul, S. M. & Holtzman, D. M. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science 295, 2264–2267 (2002).
DeMattos, R. B. et al. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 8850–8855 (2001).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02008357 (2017).
Deane, R. et al. RAGE mediates amyloid-β peptide transport across the blood–brain barrier and accumulation in brain. Nat. Med. 9, 907–913 (2003).
Yan, S. D. et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. Nature 382, 685–691 (1996).
Mackic, J. B. et al. Human blood–brain barrier receptors for Alzheimer's amyloid-β1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J. Clin. Invest. 102, 734–743 (1998).
Deane, R. et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122, 1377–1392 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02916056 (2017).
Halliday, M. R. et al. Relationship between cyclophilin A levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood–brain barrier breakdown. JAMA Neurol. 70, 1198–1200 (2013).
Conejero-Goldberg, C. et al. APOE2 enhances neuroprotection against Alzheimer's disease through multiple molecular mechanisms. Mol. Psychiatry 19, 1243–1250 (2014).
Zeuzem, S. et al. Randomised clinical trial: alisporivir combined with peginterferon and ribavirin in treatment-naive patients with chronic HCV genotype 1 infection (ESSENTIAL II). Aliment. Pharmacol. Ther. 42, 829–844 (2015).
Langford, D. et al. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J. Neuropathol. Exp. Neurol. 63, 1038–1047 (2004).
Cicchetti, F. et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann. Neurol. 76, 31–42 (2014).
Erickson, M. A. & Banks, W. A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J. Cereb. Blood Flow Metab. 33, 1500–1513 (2013).
Nuzzo, D. et al. Inflammatory mediators as biomarkers in brain disorders. Inflammation 37, 639–648 (2014).
Skoog, I. et al. A population study on blood–brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology 50, 966–971 (1998).
Janelidze, S. et al. Increased blood–brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol. Aging 51, 104–112 (2017).
Skillback, T. et al. CSF/serum albumin ratio in dementias: a cross-sectional study on 1,861 patients. Neurobiol. Aging 59, 1–9 (2017).
Blennow, K. et al. Blood–brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol. Scand. 81, 323–326 (1990).
Wallin, A., Blennow, K. & Rosengren, L. Cerebrospinal fluid markers of pathogenetic processes in vascular dementia, with special reference to the subcortical subtype. Alzheimer Dis. Assoc. Disord. 13 (Suppl. 3), S102–S105 (1999).
Blennow, K., Wallin, A., Uhlemann, C. & Gottfries, C. G. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol. Scand. 83, 187–193 (1991).
Bowman, G. L., Kaye, J. A. & Quinn, J. F. Dyslipidemia and blood–brain barrier integrity in Alzheimer's disease. Curr. Gerontol. Geriatr. Res. 2012, 184042 (2012).
Faraco, G. & Iadecola, C. Hypertension: a harbinger of stroke and dementia. Hypertension 62, 810–817 (2013).
Ivens, S. et al. TGF-β receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130, 535–547 (2007).
Braganza, O. et al. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 53, 1898–1906 (2012).
LeVine, S. M. Albumin and multiple sclerosis. BMC Neurol. 16, 47 (2016).
Silverberg, G. D. et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology 57, 1763–1766 (2001).
Craig-Schapiro, R. et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer's disease diagnosis and prognosis. PLoS ONE 6, e18850 (2011).
Hanzel, C. E. et al. Analysis of matrix metallo-proteases and the plasminogen system in mild cognitive impairment and Alzheimer's disease cerebrospinal fluid. J. Alzheimers Dis. 40, 667–678 (2014).
Pisani, V. et al. Increased blood–cerebrospinal fluid transfer of albumin in advanced Parkinson's disease. J. Neuroinflamm. 9, 188 (2012).
Liguori, C. et al. Cerebrospinal-fluid Alzheimer's disease biomarkers and blood–brain barrier integrity in a natural population of cognitive intact Parkinson's disease patients. CNS Neurol. Disord. Drug Targets 16, 339–345 (2017).
Brettschneider, J., Petzold, A., Süssmuth, S. D., Ludolph, A. C. & Tumani, H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66, 852–856 (2006).
Jessen Krut, J. et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS ONE 9, e88591 (2014).
Chen, Z. L. & Strickland, S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91, 917–925 (1997).
Mhatre, M. et al. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol. Aging 25, 783–793 (2004).
Chen, B., Cheng, Q., Yang, K. & Lyden, P. D. Thrombin mediates severe neurovascular injury during ischemia. Stroke 41, 2348–2352 (2010).
Schachtrup, C. et al. Fibrinogen inhibits neurite outgrowth via β3 integrin-mediated phosphorylation of the EGF receptor. Proc. Natl Acad. Sci. USA 104, 11814–11819 (2007).
Paul, J., Strickland, S. & Melchor, J. P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J. Exp. Med. 204, 1999–2008 (2007).
Akassoglou, K. et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc. Natl Acad. Sci. USA 101, 6698–6703 (2004).
Ryu, J. K. et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat. Commun. 6, 8164 (2015).
Bardehle, S., Rafalski, V. A. & Akassoglou, K. Breaking boundaries — coagulation and fibrinolysis at the neurovascular interface. Front. Cell. Neurosci. 9, 354 (2015).
Zhong, Z. et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 119, 3437–3449 (2009).
Sui, Y.-T., Bullock, K. M., Erickson, M. A., Zhang, J. & Banks, W. A. α-Synuclein is transported into and out of the brain by the blood–brain barrier. Peptides 62, 197–202 (2014).
Peelaerts, W. et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015).
Matsumoto, J. et al. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood–brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol. Commun. 5, 71 (2017).
Shi, M. et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 128, 639–650 (2014).
Calderón-Garcidueñas, L. et al. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimers Dis. 43, 1039–1058 (2015).
Pardridge, W. M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972 (2012).
Bray, N. Biologics: Transferrin' bispecific antibodies across the blood–brain barrier. Nat. Rev. Drug Discov. 14, 14–15 (2015).
Niewoehner, J. et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81, 49–60 (2014).
Yu, Y. J. et al. Therapeutic bispecific antibodies cross the blood–brain barrier in nonhuman primates. Sci. Transl Med. 6, 261ra154 (2014).
Yemisci, M. et al. Systemically administered brain-targeted nanoparticles transport peptides across the blood–brain barrier and provide neuroprotection. J. Cereb. Blood Flow Metab. 35, 469–475 (2015).
Burgess, A. & Hynynen, K. Microbubble-assisted ultrasound for drug delivery in the brain and central nervous system. Adv. Exp. Med. Biol. 880, 293–308 (2016).
Poon, C., McMahon, D. & Hynynen, K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology 120, 20–37 (2017).
Wang, D., Kranz-Eble, P. & De Vivo, D. C. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum. Mutat. 16, 224–231 (2000).
Alakbarzade, V. et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet. 47, 814–817 (2015).
Guemez-Gamboa, A. et al. Inactivating mutations in MFSD2A, required for ω3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 47, 809–813 (2015).
Novara, F. et al. Clinical and molecular characteristics of SLC16A2 (MCT8) mutations in three families with the Allan–Herndon–Dudley syndrome. Hum. Mutat. 38, 260–264 (2017).
Abdel-Hamid, M. S., Abdel-Salam, G. M. H., Issa, M. Y., Emam, B. A. & Zaki, M. S. Band-like calcification with simplified gyration and polymicrogyria: report of 10 new families and identification of five novel OCLN mutations. J. Hum. Genet. 62, 553–559 (2017).
Akawi, N. A. et al. Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Hum. Mutat. 34, 498–505 (2013).
Keller, A. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 (2013).
Nicolas, G. et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 80, 181–187 (2013).
Vemuri, P. & Schöll, M. Linking amyloid-β and tau deposition in Alzheimer disease. JAMA Neurol. 74, 766–768 (2017).
He, L. et al. Analysis of the brain mural cell transcriptome. Sci. Rep. 6, 35108 (2016).
Acknowledgements
The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, R01NS100459 and 5P01AG052350 in addition to the Cure Alzheimer's Fund, Alzheimer's Association and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference number 16 CVD 05.
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature search and to writing the manuscript. B.V.Z. worked closely with M.D.S. and A.P.S. to write the article and design the figures and tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Blood–brain barrier
-
(BBB). The continuous endothelial membrane of the brain vasculature, which has sealed cell-to-cell contacts and is sheathed by vascular mural cells and perivascular astrocyte end-feet; it functions to separate the circulating blood and brain compartments and strictly regulates blood-to-brain and brain-to-blood transport of solutes.
- Pericytes
-
Mural cells that wrap the brain capillary endothelium and are important for formation and maintenance of the blood–brain barrier.
- Neurodegeneration
-
Progressive neuronal dysfunction that causes neuronal degenerative changes and loss of neurons in various regions of the CNS in different neurodegenerative diseases.
- Tight junctions
-
Endothelial proteins that tightly connect brain endothelial cells and provide the anatomical blood–brain barrier with its low paracellular permeability and high transendothelial electrical resistance.
- Transmembrane diffusion
-
A type of passive transport across a cellular membrane in which the net movement of molecules occurs down their respective concentration gradients.
- Carrier-mediated transport
-
(CMT). Transport of molecules across the blood–brain barrier down their concentration gradients via specific membrane carrier proteins.
- Receptor-mediated transcytosis
-
(RMT). Transport of molecules across the blood–brain barrier in a highly specific fashion via membrane receptors that become internalized with the ligand during transendothelial transcytosis.
- Cerebrospinal fluid
-
(CSF). A fluid continually produced in the choroid plexus that flows throughout the brain's ventricular system; it functions as a clearance pathway, maintains intraventricular intracranial pressure in the brain and is often analysed to measure levels of brain-derived biomarkers of disease.
- Cerebral amyloid angiopathy
-
(CAA). In Alzheimer disease, amyloid deposition in the walls of small arteries and capillaries in the brain causes vascular degeneration and lobar microbleeds, which contribute to blood–brain barrier breakdown, infarcts, white matter changes and cognitive impairment.
- Two-hit vascular hypothesis of AD
-
Blood vessel damage is thought to be the initial insult through which blood–brain barrier dysfunction and/or diminished brain perfusion lead directly to amyloid-β (Aβ)-independent secondary neuronal injury (first hit) and Aβ accumulation (second hit) in the brain owing to faulty Aβ clearance and increased antibody production.
- E4 allele of apolipoprotein E
-
(APOE*ε4). This allele is the major genetic risk factor for sporadic late-onset Alzheimer disease.
- Dynamic contrast-enhanced (DCE) MRI
-
A dynamic MRI sequence used to quantify regional blood–brain barrier permeability to a gadolinium contrast agent.
- T2*-weighted and susceptibility-weighted imaging
-
(SWI). An MRI sequence in which haemosiderin deposits yield a hypointense signal, which enables regional in vivo measurement of cerebral microbleeds in the human brain.
- 18F-fluorodeoxyglucose
-
(FDG). An 18F-radiolabelled analogue of glucose that (unlike glucose) is not metabolized in the brain; FDG is used as a surrogate for glucose in PET studies to provide an estimate of glucose uptake by the brain across the blood–brain barrier via solute carrier family 2, facilitated glucose transporter member 1 (GLUT1).
- LDL receptor-related protein 1
-
(LRP1). The major efflux transporter for amyloid-β (Aβ) at the blood–brain barrier; it is responsible for brain-to-blood Aβ clearance.
- Verapamil
-
An 11C-radiolabelled PET ligand that enables the in vivo detection of P-glycoprotein 1 function at the blood–brain barrier in the living human brain.
- Receptor for advanced glycosylation end products
-
(RAGE). The major influx transporter of amyloid-β (Aβ) at the blood–brain barrier; it contributes to Aβ accumulation in the brain, the inflammatory response, suppression of blood flow and blood–brain barrier breakdown.
- RNA sequencing
-
A transcriptomic approach to reveal the presence and quantity of RNA transcripts in a biological sample.
- Induced pluripotent stem cells
-
(iPSCs). Adult cells reprogrammed to induce an embryonic-like pluripotent state for the purposes of inducing differentiation into a cell type of interest for research studies and/or potential therapeutic efforts.
Rights and permissions
About this article
Cite this article
Sweeney, M., Sagare, A. & Zlokovic, B. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133–150 (2018). https://doi.org/10.1038/nrneurol.2017.188
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.188
This article is cited by
-
Association between cerebrospinal fluid pressure and cognition in patients with Alzheimer’s disease and Lewy body dementia
BMC Neurology (2024)
-
Changes in lipid metabolism track with the progression of neurofibrillary pathology in tauopathies
Journal of Neuroinflammation (2024)
-
Peripheral blood amyloid-β involved in the pathogenesis of Alzheimer’s disease via impacting on peripheral innate immune cells
Journal of Neuroinflammation (2024)
-
Safety and efficacy of low-dose esketamine in laparoscopic cholecystectomy: a prospective, double-blind randomized controlled trial
BMC Anesthesiology (2024)
-
Human serum-derived α-synuclein auto-antibodies mediate NMDA receptor-dependent degeneration of CNS neurons
Journal of Neuroinflammation (2024)