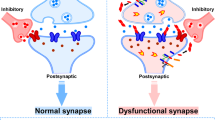
Key Points
-
Scientific advancement in neuroscience has not been effectively translated into therapies for neurological diseases. In general, 'toxin reducing' approaches (for example, lowering amyloid-β (Aβ)) thus far have not resulted in halting or delaying disease progression. A paradigm shift in the discovery of disease-modifying therapies for neurological diseases is urgently needed.
-
For neurodegenerative diseases, targeting the pathophysiology rather than the pathogenesis may be more effective to achieve therapeutic intervention. The toxin reducing approach may work if treatment starts very early on in the disease process.
-
Synapse degeneration is a major pathophysiological feature that correlates with disease progression in multiple neurodegenerative diseases. Neuronal loss is irreversible, whereas synapses can be repaired and regenerated.
-
Three aspects of synaptic physiology can be targeted: synaptic transmission, synaptic plasticity and synaptic growth. For disease-modifying therapy, synaptic plasticity and, more importantly, synaptic growth should be targeted.
-
Brain-derived neurotrophic factor (BDNF) is an exemplar of synaptic repair therapy, as it regulates all three aspects of synaptic physiology. It protects and repairs existing synapses and stimulates new synapse formation, even in the presence of various toxins.
-
In humans, the BDNF Val66Met polymorphism in conjunction with high Aβ deposits confers faster decline in Alzheimer's disease endophenotypes such as episodic memory and hippocampal volume, and therefore could be considered as a patient stratification strategy for clinical trials with enhanced sensitivity and robustness.
-
Success of a 'synaptic repair' therapy depends on whether synaptic dysfunction and synaptic repair and/or regeneration can be measured in the clinic. Efforts should be made to develop sensitive and reliable methodologies to measure synaptic function in humans in vivo.
-
Opportunities and challenges in developing BDNF–TRKB pathway-based therapies, including delivery, are discussed.
-
A combination of synaptic therapy and a more reliable and sensitive method (or methods) to measure synaptic changes may pave the way for developing disease-modifying medicines for debilitating neurological diseases. This Review highlights recent discoveries, discusses emerging concepts and proposes synapse-based therapies for treating neurodegenerative diseases.
Abstract
Increasing evidence suggests that synaptic dysfunction is a key pathophysiological hallmark in neurodegenerative disorders, including Alzheimer's disease. Understanding the role of brain-derived neurotrophic factor (BDNF) in synaptic plasticity and synaptogenesis, the impact of the BDNF Val66Met polymorphism in Alzheimer's disease-relevant endophenotypes — including episodic memory and hippocampal volume — and the technological progress in measuring synaptic changes in humans all pave the way for a 'synaptic repair' therapy for neurodegenerative diseases that targets pathophysiology rather than pathogenesis. This article reviews the key issues in translating BDNF biology into synaptic repair therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Salomon, J. A. et al. Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet 380, 2144–2162 (2013).
Brookmeyer, R., Johnson, E., Ziegler-Graham, K. & Arrighi, H. M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 3, 186–191 (2007).
Dorsey, E. R. et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386 (2007).
Alzheimer, A., Stelzmann, R. A., Schnitzlein, H. N. & Murtagh, F. R. An English translation of Alzheimer's 1907 paper, “Über eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 8, 429–431 (1995).
Gilman, S. et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562 (2005).
Mullard, A. Sting of Alzheimer's failures offset by upcoming prevention trials. Nature Rev. Drug Discov. 11, 657–660 (2012).
Herrmann, N., Chau, S. A., Kircanski, I. & Lanctot, K. L. Current and emerging drug treatment options for Alzheimer's disease: a systematic review. Drugs 71, 2031–2065 (2011).
Querfurth, H. W. & LaFerla, F. M. Alzheimer's disease. N. Engl. J. Med. 362, 329–344 (2010).
Mangialasche, F., Solomon, A., Winblad, B., Mecocci, P. & Kivipelto, M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 9, 702–716 (2010).
Perez, S. E. et al. Dimebon alters hippocampal amyloid pathology in 3xTg-AD mice. Int. J. Physiol. Pathophysiol. Pharmacol. 4, 115–127 (2012).
Zago, W. et al. Neutralization of soluble, synaptotoxic amyloid β species by antibodies is epitope specific. J. Neurosci. 32, 2696–2702 (2012).
Romberg, C., Mattson, M. P., Mughal, M. R., Bussey, T. J. & Saksida, L. M. Impaired attention in the 3xTgAD mouse model of Alzheimer's disease: rescue by donepezil (Aricept). J. Neurosci. 31, 3500–3507 (2011).
Lemere, C. A. & Masliah, E. Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nature Rev. Neurol. 6, 108–119 (2010).
Hellweg, R., Wirth, Y., Janetzky, W. & Hartmann, S. Efficacy of memantine in delaying clinical worsening in Alzheimer's disease (AD): responder analyses of nine clinical trials with patients with moderate to severe AD. Int. J. Geriatr. Psychiatry 27, 651–656 (2011).
Rodda, J., Morgan, S. & Walker, Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer's disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int. Psychogeriatr. 21, 813–824 (2009).
Lewis, D. A. & Sweet, R. A. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J. Clin. Invest. 119, 706–716 (2009). In this review, the authors emphasize the importance of separating pathogenesis from pathophysiology and suggest that therapeutic interventions for complex diseases such as schizophrenia might be more effective by focusing on pathophysiology, which is closer to clinical features.
Villemagne, V. L. et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 12, 357–367 (2013). A study that provides an estimate of changes in rates of Aβ deposition, cerebral atrophy and cognitive decline in healthy subjects, patients with MCI and patients with AD. Projections based on this longitudinal measurement suggest a protracted (∼17 years) preclinical phase of AD.
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Shankar, G. M. & Walsh, D. M. Alzheimer's disease: synaptic dysfunction and Aβ. Mol. Neurodegener. 4, 48 (2009).
Selkoe, D. J. Alzheimer's disease is a synaptic failure. Science 298, 789–791 (2002).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003). A transgenic mouse overexpressing APP Swe , PS1 M146V and tau P301L shows age-dependent relationship between Aβ, synaptic dysfunction and tangle formation. This model recapitulates the 'amyloid cascade' hypothesis in which Aβ accumulation precedes tau pathology. Impairments in synaptic transmission and LTP occur as a consequence of Aβ but prior to tau pathology.
Chapman, P. F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 2, 271–276 (1999).
Marchetti, C. & Marie, H. Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models? Rev. Neurosci. 22, 373–402 (2011).
Bittner, T. et al. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer's disease mice. PLoS ONE 5, e15477 (2010).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Dong, H., Martin, M. V., Chambers, S. & Csernansky, J. G. Spatial relationship between synapse loss and β-amyloid deposition in Tg2576 mice. J. Comp. Neurol. 500, 311–321 (2007).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Med. 14, 837–842 (2008).
Shankar, G. M. et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 (2007).
Davies, C. A., Mann, D. M., Sumpter, P. Q. & Yates, P. O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J. Neurol. Sci. 78, 151–164 (1987).
Scheff, S. W., DeKosky, S. T. & Price, D. A. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol. Aging 11, 29–37 (1990). An electron microscopic investigation of the post-mortem AD brain that showed widespread reduction in synapse number and a compensatory increase in synapse size in different layers of the cortical regions.
Masliah, E., Mallory, M., Hansen, L., DeTeresa, R. & Terry, R. D. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43, 192–197 (1993).
Scheff, S. W., Price, D. A., Schmitt, F. A. & Mufson, E. J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging 27, 1372–1384 (2006).
Scheff, S. W., Price, D. A., Schmitt, F. A., DeKosky, S. T. & Mufson, E. J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68, 1501–1508 (2007).
Chen, K. et al. Characterizing Alzheimer's disease using a hypometabolic convergence index. Neuroimage 56, 52–60 (2011).
Milnerwood, A. J. & Raymond, L. A. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 33, 513–523 (2010).
Picconi, B., Piccoli, G. & Calabresi, P. Synaptic dysfunction in Parkinson's disease. Adv. Exp. Med. Biol. 970, 553–572 (2012).
Trachtenberg, J. T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Bourne, J. N. & Harris, K. M. Nanoscale analysis of structural synaptic plasticity. Curr. Opin. Neurobiol. 22, 372–382 (2011).
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009).
Bagetta, V., Ghiglieri, V., Sgobio, C., Calabresi, P. & Picconi, B. Synaptic dysfunction in Parkinson's disease. Biochem. Soc. Trans. 38, 493–497 (2010).
Schulz-Schaeffer, W. J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 120, 131–143 (2010).
Galvin, J. E., Uryu, K., Lee, V. M. & Trojanowski, J. Q. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains α-, β-, and γ-synuclein. Proc. Natl Acad. Sci. USA 96, 13450–13455 (1999).
DiProspero, N. A. et al. Early changes in Huntington's disease patient brains involve alterations in cytoskeletal and synaptic elements. J. Neurocytol. 33, 517–533 (2004).
Mayford, M., Siegelbaum, S. A. & Kandel, E. R. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 4, a005751 (2012).
Tanaka, J. et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319, 1683–1687 (2008).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Kleim, J. A. et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature Neurosci. 9, 735–737 (2006).
Cheeran, B. et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 586, 5717–5725 (2008).
Fritsch, B. et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204 (2010). This paper demonstrates the use of a non-invasive approach — transcranial direct current stimulation — to alter BDNF secretion in human brain in vivo , suggesting that this approach improves motor learning by inducing LTP that is dependent on BDNF secretion in the motor cortex.
Ferrer, I., Goutan, E., Marin, C., Rey, M. J. & Ribalta, T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 866, 257–261 (2000).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Durany, N. et al. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer's disease brains. Int. J. Dev. Neurosci. 18, 807–813 (2000).
Hock, C., Heese, K., Hulette, C., Rosenberg, C. & Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 57, 846–851 (2000).
Phillips, H. S. et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron 7, 695–702 (1991).
Kang, H. & Schuman, E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 (1995). This is the first report to show, using rat hippocampal slices, a pharmacological effect of BDNF on synaptic transmission.
Messaoudi, E., Bårdsen, K., Srebro, B. & Bramham, C. R. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J. Neurophysiol. 79, 496–499 (1998).
Figurov, A., Pozzo-Miller, L. D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996). This is the first report to show a pharmacological effect of BDNF on LTP.
Patterson, S. L. et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145 (1996).
Tanaka, T., Saito, H. & Matsuki, N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 17, 2959–2966 (1997).
Frerking, M., Malenka, R. C. & Nicoll, R. A. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J. Neurophysiol. 80, 3383–3386 (1998).
Gottschalk, W., Pozzo-Miller, L. D., Figurov, A. & Lu, B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 18, 6830–6839 (1998).
Akaneya, Y., Tsumoto, T., Kinoshita, S. & Hatanaka, H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J. Neurosci. 17, 6707–6716 (1997).
Huber, K. M., Sawtell, N. B. & Bear, M. F. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology 37, 571–579 (1998).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 8856–8860 (1995).
Monteggia, L. M. et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl Acad. Sci. USA 101, 10827–10832 (2004).
Minichiello, L. et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24, 401–414 (1999).
Xu, B. et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 20, 6888–6897 (2000).
Ji, Y. et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nature Neurosci. 13, 302–309 (2010). This study resolves a long-term dispute: whether BDNF enhances basal synaptic transmission. It demonstrates that a gradual increase in BDNF concentration facilitates LTP, whereas a rapid rise of BDNF concentration increases basal synaptic transmission. Thus, a single extracellular factor could induce distinct signalling and functions based on how it is delivered.
Lu, Y., Christian, K. & Lu, B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 89, 312–323 (2008).
Korte, M., Kang, H., Bonhoeffer, T. & Schuman, E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology 37, 553–559 (1998).
Balkowiec, A. & Katz, D. M. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J. Neurosci. 20, 7417–7423 (2000).
Balkowiec, A. & Katz, D. M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 22, 10399–10407 (2002).
Nagappan, G. et al. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc. Natl Acad. Sci. USA 106, 1267–1272 (2009).
Patterson, S. L., Grover, L. M., Schwartzkroin, P. A. & Bothwell, M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron 9, 1081–1088 (1992).
Kang, H., Welcher, A. A., Shelton, D. & Schuman, E. M. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 19, 653–664 (1997).
Chen, G., Kolbeck, R., Barde, Y. A., Bonhoeffer, T. & Kossel, A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J. Neurosci. 19, 7983–7990 (1999).
Pang, P. T. et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491 (2004).
Barco, A. et al. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48, 123–137 (2005).
Lu, Y. et al. TrkB as a potential synaptic and behavioral tag. J. Neurosci. 31, 11762–11771 (2011).
Park, H. & Poo, M. M. Neurotrophin regulation of neural circuit development and function. Nature Rev. Neurosci. 14, 7–23 (2013). A recent review summarizing progress in the understanding of the cellular and molecular mechanisms underlying BDNF regulation of neural circuits in the brain.
Cohen-Cory, S. & Fraser, S. E. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature 378, 192–196 (1995).
McAllister, A. K., Lo, D. C. & Katz, L. C. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 (1995).
Cabelli, R. J., Hohn, A. & Shatz, C. J. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science 267, 1662–1666 (1995).
Tyler, W. J. & Pozzo-Miller, L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J. Physiol. 553, 497–509 (2003). An important study that clearly demonstrates the pharmacological effect of BDNF on synaptic growth; that is, it found an increase in the number of dendritic spines.
Tartaglia, N. et al. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J. Biol. Chem. 276, 37585–37593 (2001).
Horch, H. W., Kruttgen, A., Portbury, S. D. & Katz, L. C. Destabilization of cortical dendrites and spines by BDNF. Neuron 23, 353–364 (1999).
Pozzo-Miller, L. D. et al. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 19, 4972–4983 (1999).
Otal, R., Martinez, A. & Soriano, E. Lack of TrkB and TrkC signaling alters the synaptogenesis and maturation of mossy fiber terminals in the hippocampus. Cell Tissue Res. 319, 349–358 (2005).
Aguado, F. et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development 130, 1267–1280 (2003).
Singh, B. et al. Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J. Neurosci. 26, 7189–7200 (2006).
Ma, Y. L., Wang, H. L., Wu, H. C., Wei, C. L. & Lee, E. H. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience 82, 957–967 (1998).
Mu, J. S., Li, W. P., Yao, Z. B. & Zhou, X. F. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 835, 259–265 (1999).
Linnarsson, S., Bjorklund, A. & Ernfors, P. Learning deficit in BDNF mutant mice. Eur. J. Neurosci. 9, 2581–2587 (1997).
Chen, X. et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron 46, 13–21 (2005).
Johnson, A. W. et al. The brain-derived neurotrophic factor receptor TrkB is critical for the acquisition but not expression of conditioned incentive value. Eur. J. Neurosci. 28, 997–1002 (2008).
Alonso, M., Vianna, M. R., Izquierdo, I. & Medina, J. H. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell. Mol. Neurobiol. 22, 663–674 (2002).
Koponen, E. et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB–PLCγ pathway, reduced anxiety, and facilitated learning. Mol. Cell Neurosci. 26, 166–181 (2004).
Soliman, F. et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327, 863–866 (2010).
Martinowich, K. & Lu, B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83 (2008).
Martinowich, K., Manji, H. & Lu, B. New insights into BDNF function in depression and anxiety. Nature Neurosci. 10, 1089–1093 (2007).
Rakofsky, J. J., Ressler, K. J. & Dunlop, B. W. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol. Psychiatry 17, 22–35 (2012).
Nagahara, A. H. & Tuszynski, M. H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature Rev. Drug Discov. 10, 209–219 (2011). A comprehensive review that discusses the role of BDNF in multiple neurological and psychiatric disorders and its therapeutic use thereof.
Kemppainen, S. et al. Impaired TrkB receptor signaling contributes to memory impairment in APP/PS1 mice. Neurobiol. Aging 33, 1122.e23–1122.e39 (2012).
Nagahara, A. H. et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature Med. 15, 331–337 (2009). A systematic investigation of the neuroprotective effects of BDNF in APP transgenic mice, aged rats and brain lesioned non-human primates, using a therapeutic modality that ameliorated synapse loss, neuronal atrophy and restored cognitive deficits without directly altering the level of toxins (specifically, amyloid).
Zeng, Y., Zhao, D. & Xie, C. W. Neurotrophins enhance CaMKII activity and rescue amyloid-β-induced deficits in hippocampal synaptic plasticity. J. Alzheimers Dis. 21, 823–831 (2010).
Egan, M. F. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003). A pioneering study linking a human genetic polymorphism, BDNF Val66Met, to a reduction in activity-dependent BDNF secretion. This study explained the changes in hippocampal function and episodic memory associated with the polymorphism and triggered many subsequent studies on the impact of the BDNF Val66Met polymorphism on various cognitive functions and brain disorders in humans.
Chen, Z. Y. et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143 (2006).
Pezawas, L. et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 24, 10099–10102 (2004).
Szeszko, P. R. et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry 10, 631–636 (2005).
Yang, X. et al. Impact of brain-derived neurotrophic factor Val66Met polymorphism on cortical thickness and voxel-based morphometry in healthy Chinese young adults. PLoS ONE 7, e37777 (2012).
Sanchez, M. M. et al. BDNF polymorphism predicts the rate of decline in skilled task performance and hippocampal volume in healthy individuals. Transl. Psychiatry 1, e51 (2011).
Molendijk, M. L. et al. A systematic review and meta-analysis on the association between BDNF val66met and hippocampal volume — a genuine effect or a winners curse? Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 731–740 (2012).
Campbell, S., Marriott, M., Nahmias, C. & MacQueen, G. M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry 161, 598–607 (2004).
Videbech, P. & Ravnkilde, B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry 161, 1957–1966 (2004).
Zivadinov, R. et al. Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum. Mol. Genet. 16, 2659–2668 (2007).
Voineskos, A. N. et al. The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Arch. Gen. Psychiatry 68, 198–206 (2011).
Dennis, N. A. et al. Brain-derived neurotrophic factor val66met polymorphism and hippocampal activation during episodic encoding and retrieval tasks. Hippocampus 21, 980–989 (2011).
Putcha, D. et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J. Neurosci. 31, 17680–17688 (2011).
Yassa, M. A. et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51, 1242–1252 (2010).
Dempster, E. et al. Association between BDNF val66 met genotype and episodic memory. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 73–75 (2005).
Ho, B. C. et al. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch. Gen. Psychiatry 63, 731–740 (2006).
Miyajima, F. et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 7, 411–417 (2008).
Schofield, P. R. et al. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: evidence from cognition, the P300 and fronto-hippocampal systems. Biol. Psychol. 80, 176–188 (2009).
Mandelman, S. D. & Grigorenko, E. L. BDNF Val66Met and cognition: all, none, or some? A meta-analysis of the genetic association. Genes Brain Behav. 11, 127–136 (2012).
Cirillo, J., Hughes, J., Ridding, M., Thomas, P. Q. & Semmler, J. G. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. Eur. J. Neurosci. 36, 2640–2649 (2012).
Antal, A. et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 3, 230–237 (2010).
Witte, A. V. et al. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J. Neurosci. 32, 4553–4561 (2012).
Li Voti, P. et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp. Brain Res. 212, 91–99 (2011).
Di Lazzaro, V. et al. The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. J. Neural Transm. 119, 1499–1506 (2012).
Gatt, J. M. et al. Association between BDNF Val66Met polymorphism and trait depression is mediated via resting EEG alpha band activity. Biol. Psychol. 79, 275–284 (2008).
Beste, C. et al. The role of the BDNF Val66Met polymorphism for the synchronization of error-specific neural networks. J. Neurosci. 30, 10727–10733 (2010).
Bian, J. T., Zhang, J. W., Zhang, Z. X. & Zhao, H. L. Association analysis of brain-derived neurotrophic factor (BDNF) gene 196 A/G polymorphism with Alzheimer's disease (AD) in mainland Chinese. Neurosci. Lett. 387, 11–16 (2005).
Borroni, B. et al. Role of BDNF Val66Met functional polymorphism in Alzheimer's disease-related depression. Neurobiol. Aging 30, 1406–1412 (2009).
Lim, Y. Y. et al. Modulation of Aβ amyloid-related cognitive decline by brain-derived neurotrophic factor Val66Met polymorphism in preclinical Alzheimer's disease. Neurobiol. Aging (in the press). A longitudinal study demonstrating an epistatic relationship between the BDNF Val66Met polymorphism and amyloid deposition in both preclinical and clinical phases of AD. Subjects with high levels of Aβ combined with the BDNFMet polymorphism showed a faster decline in hippocampal volume and episodic memory.
Karamohamed, S. et al. BDNF genetic variants are associated with onset age of familial Parkinson disease: GenePD Study. Neurology 65, 1823–1825 (2005).
Momose, Y. et al. Association studies of multiple candidate genes for Parkinson's disease using single nucleotide polymorphisms. Ann. Neurol. 51, 133–136 (2002).
Parsian, A., Sinha, R., Racette, B., Zhao, J. H. & Perlmutter, J. S. Association of a variation in the promoter region of the brain-derived neurotrophic factor gene with familial Parkinson's disease. Parkinsonism Relat. Disord. 10, 213–219 (2004).
Guerini, F. R. et al. BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson's disease. Eur. J. Neurol. 16, 1240–1245 (2009).
Liu, J. et al. Brain-derived neurotrophic factor (BDNF) genetic polymorphism greatly increases risk of leucine-rich repeat kinase 2 (LRRK2) for Parkinson's disease. Parkinsonism Relat. Disord. 18, 140–143 (2012).
Ninan, I. et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 30, 8866–8870 (2010).
Liu, R. J. et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry 71, 996–1005 (2012).
Cao, L. et al. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr. Biol. 17, 911–921 (2007).
Thoenen, H. & Sendtner, M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nature Neurosci. 5, 1046–1050 (2002).
Bradley, W. G. Miami, F. L. & the BDNF Trial Group. A PhaseI/II study of recombinant human brain-derived neurotrophic factor in patients with amyotrophic lateral sclerosis. Ann. Neurol. 38, 971 (1995).
[No authors listed.] A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III). Neurology 52, 1427–1433 (1999).
Ochs, G. et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor. Neuron Disord. 1, 201–206 (2000).
Apfel, S. C. Is the therapeutic application of neurotrophic factors dead? Ann. Neurol. 51, 8–11 (2002).
Henriques, A., Pitzer, C. & Schneider, A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front. Neurosci. 4, 32 (2010).
Morgan, P. et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today 17, 419–424 (2012).
Dittrich, F. et al. Pharmacokinetics of intrathecally applied BDNF and effects on spinal motoneurons. Exp. Neurol. 141, 225–239 (1996).
Lampe, K. J., Kern, D. S., Mahoney, M. J. & Bjugstad, K. B. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: protein distribution and the glial response. J. Biomed. Mater. Res. A 96, 595–607 (2011).
Bertram, J. P., Rauch, M. F., Chang, K. & Lavik, E. B. Using polymer chemistry to modulate the delivery of neurotrophic factors from degradable microspheres: delivery of BDNF. Pharm. Res. 27, 82–91 (2010).
Boado, R. J., Zhang, Y. & Pardridge, W. M. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood–brain barrier. Biotechnol. Bioeng. 97, 1376–1386 (2007).
Zhang, Y. & Pardridge, W. M. Conjugation of brain-derived neurotrophic factor to a blood–brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 889, 49–56 (2001).
Yu, Y. J. et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 3, 84ra44 (2011).
Demeule, M. et al. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 324, 1064–1072 (2008).
Gaillard, P. J., Visser, C. C. & de Boer, A. G. Targeted delivery across the blood–brain barrier. Expert Opin. Drug Deliv. 2, 299–309 (2005).
Muruganandam, A., Tanha, J., Narang, S. & Stanimirovic, D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood–brain barrier endothelium. FASEB J. 16, 240–242 (2002).
Geral, C., Angelova, A. & Lesieur, S. From molecular to nanotechnology strategies for delivery of neurotrophins: emphasis on brain-derived neurotrophic factor (BDNF). Pharmaceutics 5, 127–167 (2013). A comprehensive review describing the approaches to deliver BDNF and other proteins into the CNS using nanotechnology.
Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 37, 48–57 (2010).
Alcala-Barraza, S. R. et al. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target 18, 179–190 (2010).
Jiang, Y. et al. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience 172, 398–405 (2011).
Poduslo, J. F. & Curran, G. L. Permeability at the blood–brain and blood–nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 36, 280–286 (1996).
Sakane, T. & Pardridge, W. M. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 14, 1085–1091 (1997).
Jang, S. W. et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl Acad. Sci. USA 107, 2687–2692 (2010).
Massa, S. M. et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 120, 1774–1785 (2010).
Qian, M. D. et al. Novel agonist monoclonal antibodies activate TrkB receptors and demonstrate potent neurotrophic activities. J. Neurosci. 26, 9394–9403 (2006).
Lin, J. C. et al. Appetite enhancement and weight gain by peripheral administration of TrkB agonists in non-human primates. PLoS ONE 3, e1900 (2008).
O'Leary, P. D. & Hughes, R. A. Design of potent peptide mimetics of brain-derived neurotrophic factor. J. Biol. Chem. 278, 25738–25744 (2003).
Fletcher, J. M. & Hughes, R. A. Novel monocyclic and bicyclic loop mimetics of brain-derived neurotrophic factor. J. Pept. Sci. 12, 515–524 (2006).
Cardenas- Aguayo Mdel, C., Kazim, S. F., Grundke-Iqbal, I. & Iqbal, K. Neurogenic and neurotrophic effects of BDNF peptides in mouse hippocampal primary neuronal cell cultures. PLoS ONE 8, e53596 (2013).
Jang, S. W. et al. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS ONE 5, e11528 (2010).
Lanz, T. A. et al. Development of an assay to identify activators of TrkB signaling using human induced pluripotent stem cell derived neurons. Soc. Neurosci. Abstr. 435.08 (14 Nov 2011, Washington D.C.).
Tsao, D. et al. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology 149, 1038–1048 (2008).
Xu, L., Zhang, Y., Cohen, S. B. & DiPetrillo, K. TrkB agonist antibody dose-dependently raises blood pressure in mice with diet-induced obesity. Am. J. Hypertens. 23, 732–736 (2010).
Zoladz, J. A. & Pilc, A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J. Physiol. Pharmacol. 61, 533–541 (2010).
Knaepen, K., Goekint, M., Heyman, E. M. & Meeusen, R. Neuroplasticity — exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 40, 765–801 (2010).
Adlard, P. A., Perreau, V. M. & Cotman, C. W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging 26, 511–520 (2005).
Nibuya, M., Morinobu, S. & Duman, R. S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15, 7539–7547 (1995).
Altar, C. A., Whitehead, R. E., Chen, R., Wortwein, G. & Madsen, T. M. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry 54, 703–709 (2003).
Saarelainen, T. et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 23, 349–357 (2003).
Rantamaki, T. et al. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS ONE 6, e20567 (2011).
Zarate, C. A. Jr et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006).
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011). This study shows that low doses of NMDA receptor antagonists such as ketamine rapidly increase the synthesis of BDNF in the mouse brain through post-transcriptional mechanisms.
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010).
Marvanova, M. et al. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell Neurosci. 18, 247–258 (2001).
Leyhe, T., Stransky, E., Eschweiler, G. W., Buchkremer, G. & Laske, C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. 258, 124–128 (2008).
Autio, H. et al. Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology 61, 1291–1296.
Alonso, M., Medina, J. H. & Pozzo-Miller, L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. 11, 172–178 (2004).
Hashimoto, R. et al. Effect of the brain-derived neurotrophic factor and the apolipoprotein E polymorphisms on disease progression in preclinical Alzheimer's disease. Genes Brain Behav. 8, 43–52 (2009).
Adamczuk, K. et al. Polymorphism of brain derived neurotrophic factor influences β amyloid load in cognitively intact apolipoprotein Eɛ4 carriers. Neuroimage Clin. (in the press).
Blin, O., Davies, C. H. & Lu, B. Clinical innovation for neurodegenerative diseases. Clin. Invest. 2, 663–665 (2012).
Huang, Y. & Morozov, A. Hippocampal deletion of BDNF gene attenuates gamma oscillations in area CA1 by up-regulating 5-HT3 receptor. PLoS ONE 6, e16480 (2011).
Zheng, K. et al. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc. Natl Acad. Sci. USA 108, 17201–17206 (2011).
Rossini, P. M. et al. Conversion from mild cognitive impairment to Alzheimer's disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 143, 793–803 (2006).
Lai, C. L., Lin, R. T., Liou, L. M. & Liu, C. K. The role of event-related potentials in cognitive decline in Alzheimer's disease. Clin. Neurophysiol. 121, 194–199 (2010).
Miller, G. Alzheimer's research. Stopping Alzheimer's before it starts. Science 337, 790–792 (2012).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 367, 795–804 (2012).
Reiman, E. M. et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 11, 1048–1056 (2012).
Alonso Canovas, A. et al. Dopaminergic agonists in Parkinson's disease. Neurologia 2 Jul 2011 (doi:10.1016/j.nrl.2011.04.012).
Tong, L. & Perez-Polo, R. Brain-derived neurotrophic factor (BDNF) protects cultured rat cerebellar granule neurons against glucose deprivation-induced apoptosis. J. Neural Transm. 105, 905–914 (1998).
Ferenz, K. B. et al. Nerve growth factor and brain-derived neurotrophic factor but not granulocyte colony-stimulating factor, nimodipine and dizocilpine, require ATP for neuroprotective activity after oxygen-glucose deprivation of primary neurons. Brain Res. 1448, 20–26 (2012).
Lee, B. et al. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 108, 1251–1265 (2009).
Lindholm, D., Dechant, G., Heisenberg, C. P. & Thoenen, H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur. J. Neurosci. 5, 1455–1464 (1993).
Arancibia, S. et al. Protective effect of BDNF against β-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 31, 316–326 (2008).
Ferrer, I. et al. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 101, 229–238 (2001).
Müller, H. D. et al. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke 39, 1012–1021 (2008).
Frim, D. M. et al. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl Acad. Sci. USA 91, 5104–5108 (1994).
Shults, C. W., Kimber, T. & Altar, C. A. BDNF attenuates the effects of intrastriatal injection of 6-hydroxydopamine. Neuroreport 6, 1109–1112 (1995).
Tandon, P., Yang, Y., Das, K., Holmes, G. L. & Stafstrom, C. E. Neuroprotective effects of brain-derived neurotrophic factor in seizures during development. Neuroscience 91, 293–303 (1999).
Luikart, B. W. et al. TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J. Neurosci. 25, 3774–3786 (2005).
Cattaneo, A., Capsoni, S. & Paoletti, F. Towards non invasive nerve growth factor therapies for Alzheimer's disease. J. Alzheimers Dis. 15, 255–283 (2008).
Petryshen, T. L. et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry 15, 810–815 (2010).
Shimizu, E., Hashimoto, K. & Iyo, M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 126B, 122–123 (2004).
Ramasamy, D. P. et al. Effect of Met66 allele of the BDNF rs6265 SNP on regional gray matter volumes in patients with multiple sclerosis: a voxel-based morphometry study. Pathophysiology 18, 53–60 (2011).
Hall, D., Dhilla, A., Charalambous, A., Gogos, J. A. & Karayiorgou, M. Sequence variants of the brain-derived neurotrophic factor (BDNF) gene are strongly associated with obsessive-compulsive disorder. Am. J. Hum. Genet. 73, 370–376 (2003).
Geller, B. et al. Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am. J. Psychiatry 161, 1698–1700 (2004).
Martinez, A. et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J. Neurosci. 18, 7336–7350 (1998).
Jang, S. W. et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl Acad. Sci. USA 107, 3876–3881 (2010).
Lee, F. S. & Chao, M. V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl Acad. Sci. USA 98, 3555–3560 (2001).
Lee, F. S., Rajagopal, R., Kim, A. H., Chang, P. C. & Chao, M. V. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 277, 9096–9102 (2002).
Swift, J. L. et al. Quantification of receptor tyrosine kinase transactivation through direct dimerization and surface density measurements in single cells. Proc. Natl Acad. Sci. USA 108, 7016–7021 (2011).
Huang, Y. Z., Pan, E., Xiong, Z. Q. & McNamara, J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber–CA3 pyramid synapse. Neuron 57, 546–558 (2008).
Fletcher, J. M. et al. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor. J. Biol. Chem. 283, 33375–33383 (2008).
Peter, J.-C. et al. Anti-trkb antibodies as pharmacological tools to study the function of the TrkB receptor and its role in the regulation of food intake. Pharmacologia 4, 1–14 (2013).
Huang, Y. Z. et al. RNA aptamer-based functional ligands of the neurotrophin receptor, TrkB. Mol. Pharmacol. 82, 623–635 (2012).
Lauterborn, J. C., Lynch, G., Vanderklish, P., Arai, A. & Gall, C. M. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J. Neurosci. 20, 8–21 (2000).
Lauterborn, J. C. et al. Chronic elevation of brain-derived neurotrophic factor by ampakines. J. Pharmacol. Exp. Ther. 307, 297–305 (2003).
Lauterborn, J. C. et al. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience 159, 283–295 (2009).
Legutko, B., Li, X. & Skolnick, P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 40, 1019–1027 (2001).
Nibuya, M., Nestler, E. J. & Duman, R. S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 16, 2365–2372 (1996).
Deogracias, R. et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 109, 14230–14235 (2012).
Apostol, B. L. et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol. Cell Neurosci. 39, 8–20 (2008).
Rasmussen, P. et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 94, 1062–1069 (2009).
Kotani, S., Yamauchi, T., Teramoto, T. & Ogura, H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 142, 505–514 (2006).
Wang, Z. F., Tang, L. L., Yan, H., Wang, Y. J. & Tang, X. C. Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol. Biochem. Behav. 83, 603–611 (2006).
Borrell-Pages, M. et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Invest. 116, 1410–1424 (2006).
Acknowledgements
We thank our colleagues at GlaxoSmithKline and at various academic institutes for their critical comments and suggestions during our discussions on the topic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Disease-modifying therapies
-
Medical therapies that address the cause of the disease either directly or indirectly and thereby modify the course of the disease: that is, slow down, halt or reverse disease progression.
- Symptomatic treatment
-
A medical therapy that only relieves or controls the disease symptoms but not its cause per se.
- Phase III
-
A trial conducted to gather new information about the safety and effectiveness of a particular therapy in a larger group of patients than that used in Phase II clinical trials.
- Synaptic plasticity
-
Activity-dependent modulation of synaptic structure and/or function.
- Synaptogenesis
-
The formation of new synapses between neurons, and the maturation and stabilization of existing synapses. It is also termed synaptic growth.
- Early-phase LTP
-
(E-LTP). Early-phase long-term potentiation (LTP) is a sustained increase in synaptic efficacy that is induced by brief, high-frequency tetanic stimulation and lasts for 1–2 hours. It does not require gene transcription or protein synthesis.
- Late-phase LTP
-
(L-LTP). Late-phase long-term potentiation (LTP) is a long-lasting increase in synaptic efficacy that is induced by strong, multiple tetanic stimuli and lasts for several hours or even days. It is both transcription- and translation-dependent and is often accompanied by morphological changes at the stimulated synapses.
- Paired associative stimulation
-
A neurophysiological paradigm that involves stimulation of the median peripheral nerve followed by transcranial magnetic stimulation of the contralateral motor cortex. This paradigm has been successfully used to induce plasticity changes in the human motor cortex.
- Gene dosage
-
A linear relationship between the number of genes (or alleles), the gene (or allele) product and the resulting effect (the phenotype).
- Epistatic
-
The effect of one gene or gene product influencing the effect of other genes or gene products.
- Endophenotypes
-
Intrinsic phenotypes that are relevant to a disease but not evident without a test. A good endophenotype must be tightly associated with the disease and display familial association even in non-diseased relatives with a higher odds ratio than in the general population.
- Phase I
-
Phase I trials are typically conducted in healthy volunteers or in patients in a closely monitored clinic to evaluate safety, tolerability and pharmacokinetics of a new investigational drug.
- Forced vital capacity
-
The amount of air that can be forcibly exhaled from the lungs after a deep breath, which can be measured with a spirometer.
- Phase II
-
A trial conducted primarily to evaluate the effectiveness of a drug in people who have a certain disease or condition. Safety continues to be evaluated in the clinical setting. Initial Phase II efficacy studies are also referred to as proof-of-concept studies.
- ALSFRS
-
Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R is the revised version) is a validated measure (scores 0–48) that aids the assessment of disability of the patients with motor neuron diseases based on a questionnaire that asks about daily activities and how much help the patients need along with disease-specific symptoms.
- Nanoparticle
-
A microscopic particle with a diameter of less than 100 nm. Here, it refers to the liposomes or exosomes that carry drug substances into the brain.
- Trojan horse
-
A strategy to deliver drugs to target sites that are normally inaccessible. The drug is fused to a molecule or encapsulated in a cell or nanoparticle that can readily cross the blood–brain barrier.
- Nose-to-brain
-
Delivery or transport of drugs, cells or cargoes into the brain intranasally through the olfactory or trigeminal neuronal pathway. This delivery route limits systemic exposure and bypasses the blood–brain barrier.
Rights and permissions
About this article
Cite this article
Lu, B., Nagappan, G., Guan, X. et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14, 401–416 (2013). https://doi.org/10.1038/nrn3505
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3505
This article is cited by
-
Targeting synapse function and loss for treatment of neurodegenerative diseases
Nature Reviews Drug Discovery (2024)
-
Rapid ejaculator rats are more susceptible to anxiety compared with normal ejaculator rats
International Journal of Impotence Research (2024)
-
Sericin Improves Memory Impairment Via Activation of the PKA-CREB-BDNF Signaling Pathway and Suppression of Oxidative Stress in Ovariectomized Mice
Neurochemical Research (2024)
-
Loss of TDP-43 function underlies hippocampal and cortical synaptic deficits in TDP-43 proteinopathies
Molecular Psychiatry (2023)
-
Time-dependent homeostatic mechanisms underlie brain-derived neurotrophic factor action on neural circuitry
Communications Biology (2023)