Key Points
-
Summary points that barrier scientists and neuroscientists need to collaborate on:
-
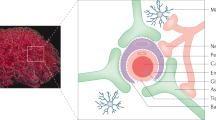
The neurovascular unit (NVU; comprising cellular and acellular elements of brain vessels, parenchymal cells and peripheral immune cells) incorporates three main functionalities — blood–brain barrier, neuroimmune axis and regulation of the cerebral blood flow — that are tightly integrated in brain physiology and play a part in the pathogenesis of numerous neurological diseases.
-
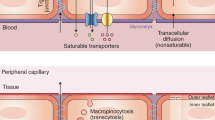
Exchange of information, nutrients and molecules between systemic and central compartments is controlled by the myriad of NVU transporters, which become dysfunctional in brain diseases such as epilepsy, brain tumours and Alzheimer's disease.
-
Targeting the intracellular signalling pathways that regulate selective blood–brain barrier transporters can potentially be used to enhance brain drug delivery, protect the brain from xenobiotics and prevent the pathogenesis and/or slow the progression of CNS diseases.
-
Neurogenesis and angiogenesis are co-regulated in embryonic and adult brains and are often controlled by the same classes of mediators. Novel methods for co-ordinated stimulation of both neuronal and vascular regeneration will be essential to develop successful brain repair strategies.
-
Progress in understanding and treating brain disease is contingent upon better understanding of the integral function of the NVU in disease, advancing the means to interrogate molecular and functional aspects of the NVU, and the development of strategies to deliver therapeutics across the blood–brain barrier.
-
New high resolution imaging techniques are providing stubstantial advances in the blood–brain barrier field and have a powerful potential for further progress. In particular, in vivo two-photon imaging studies of interactions of glial cells and blood cells with the blood–brain barrier are required to compose an integrated picture of blood–brain barrier regulation and function.
Abstract
The delivery of many potentially therapeutic and diagnostic compounds to specific areas of the brain is restricted by brain barriers, of which the most well known are the blood–brain barrier (BBB) and the blood–cerebrospinal fluid (CSF) barrier. Recent studies have shown numerous additional roles of these barriers, including an involvement in neurodevelopment, in the control of cerebral blood flow, and — when barrier integrity is impaired — in the pathology of many common CNS disorders such as Alzheimer's disease, Parkinson's disease and stroke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Selkoe, D. J. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann. Intern. Med. 140, 627–638 (2004).
Zlokovic, B. V. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 28, 202–208 (2005).
Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 120, 287–296 (2010).
Kortekaas, R. et al. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 57, 176–179 (2005).
Gold, R., Linington, C. & Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129, 1953–1971 (2006).
Hemmer, B., Cepok, S., Zhou, D. & Sommer, N. Multiple sclerosis — a coordinated immune attack across the blood brain barrier. Curr. Neurovasc. Res. 1, 141–150 (2004).
Shlosberg, D., Benifla, M., Kaufer, D. & Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature Rev. Neurol. 6, 393–403 (2010).
Barzo, P., Marmarou, A., Fatouros, P., Hayasaki, K. & Corwin, F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg. 87, 900–907 (1997).
Jain, R. K. et al. Angiogenesis in brain tumours. Nature Rev. Neurosci. 8, 610–622 (2007).
Papadopoulos, M. C. et al. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol. Appl. Neurobiol. 27, 384–395 (2001).
Moskowitz, M. A., Lo, E. H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Belayev, L., Busto, R., Zhao, W. & Ginsberg, M. D. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 739, 88–96 (1996).
Benchenane, K., Lopez-Atalaya, J. P., Fernandez-Monreal, M., Touzani, O. & Vivien, D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 27, 155–160 (2004).
Kuroiwa, T., Ting, P., Martinez, H. & Klatzo, I. The biphasic opening of the blood–brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 68, 122–129 (1985).
Navarro Mora, G. et al. Does pilocarpine-induced epilepsy in adult rats require status epilepticus? PLoS ONE 4, e5759 (2009).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nature Neurosci. 9, 260–267 (2006).
Iadecola, C. & Nedergaard, M. Glial regulation of the cerebral microvasculature. Nature Neurosci. 10, 1369–1376 (2007). A comprehensive review of the role of astrocytes in the control of cerebral blood flow, highlighting some of the areas that require more research.
Stenman, J. M. et al. Canonical Wnt signalling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 (2008). This study provides evidence of the brain-specific role of canonical Wnt signalling in differentiation of highly specialized CNS vasculature, including BBB phenotype.
Abbott, N. J., Ronnback, L. & Hansson, E. Astrocyte-endothelial interactions at the blood–brain barrier. Nature Rev. Neurosci. 7, 41–53 (2006). This review gives a detailed picture of astrocyte–endothelial interactions and signalling.
Neuwelt, E. et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 7, 84–96 (2008).
Perea, G., Navarrete, M. & Araque, A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431 (2009).
Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215 (1999).
Grotta, J. C., Jacobs, T. P., Koroshetz, W. J. & Moskowitz, M. A. Stroke program review group: an interim report. Stroke 39, 1364–1370 (2008).
Barres, B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008). A concise and complete primer on the role of astrocytes in brain function, with emphasis on their role in BBB function.
Leybaert, L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J. Cereb. Blood Flow Metab. 25, 2–16 (2005).
Lok, J. et al. Cell–cell signalling in the neurovascular unit. Neurochem. Res. 32, 2032–2045 (2007).
Daneman, R. et al. Wnt/beta-catenin signalling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl Acad. Sci. USA 106, 641–646 (2009).
McCarty, J. H. Cell adhesion and signalling networks in brain neurovascular units. Curr. Opin. Hematol. 16, 209–214 (2009).
MacAulay, N. & Zeuthen, T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168, 941–956 (2010).
Miller, D. S. Regulation of P-glycoprotein and other ABC drug transporters at the blood–brain barrier. Trends Pharmacol. Sci. 31, 246–254 (2010). A comprehensive, up-to-date survey of efflux transporters at the BBB, with a review of current knowledge of Pgp (the best studied efflux transporter) mechanisms.
Kimelberg, H. K. & Nedergaard, M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7, 338–353 (2010).
Zlokovic, B. V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008). A very detailed analysis of BBB alterations, including its various transporters, in neurodegenerative disease.
Pedersen, S. F., O'Donnell, M. E., Anderson, S. E. & Cala, P. M. Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl− cotransport in the heart, brain, and blood. Am. J. Physiol. Regul. Integr Comp. Physiol. 291, R1–R25 (2006).
Persidsky, Y., Ramirez, S. H., Haorah, J. & Kanmogne, G. D. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 1, 223–236 (2006).
Jin, R., Yang, G. & Li, G. Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 38, 376–385 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Madri, J. A. Modeling the neurovascular niche: implications for recovery from CNS injury. J. Physiol. Pharmacol. 60 (Suppl. 4), 95–104 (2009).
Ohtsuki, S. & Terasaki, T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Res. 24, 1745–1758 (2007).
Enerson, B. E. & Drewes, L. R. The rat blood–brain barrier transcriptome. J. Cereb. Blood Flow Metab. 26, 959–973 (2006).
Pardridge, W. M. Molecular biology of the blood–brain barrier. Methods Mol. Med. 89, 385–399 (2003).
Torres, G. E. & Amara, S. G. Glutamate and monoamine transporters: new visions of form and function. Curr. Opin. Neurobiol. 17, 304–312 (2007).
Locher, K. P. Review. Structure and mechanism of ATP-binding cassette transporters. Phil. Trans. R. Soc. Lond. B 364, 239–245 (2009).
Melzer, N., Torres-Salazar, D. & Fahlke, C. A dynamic switch between inhibitory and excitatory currents in a neuronal glutamate transporter. Proc. Natl Acad. Sci. USA 102, 19214–19218 (2005).
Bunch, L., Erichsen, M. N. & Jensen, A. A. Excitatory amino acid transporters as potential drug targets. Expert Opin. Ther. Targets. 13, 719–731 (2009).
Bridges, R. J. & Esslinger, C. S. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol. Ther. 107, 271–285 (2005).
Gonzalez, M. I. & Robinson, M. B. Neurotransmitter transporters: why dance with so many partners? Curr. Opin. Pharmacol. 4, 30–35 (2004).
Chen, L. M., Haddad, G. G. & Boron, W. F. Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Res. 1238, 85–92 (2008).
Bevensee, M. O. & Boron, W. F. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Res. 1193, 143–152 (2008).
Chen, L. M. et al. Expression and localization of Na-driven Cl–HCO3– exchanger (SLC4A8) in rodent CNS. Neuroscience 153, 162–174 (2008).
Amiry-Moghaddam, M. et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl Acad. Sci. USA 100, 2106–2111 (2003).
Benfenati, V. & Ferroni, S. Water transport between CNS compartments: functional and molecular interactions between aquaporins and ion channels. Neuroscience 168, 926–940 (2010).
Speake, T., Freeman, L. J. & Brown, P. D. Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim. Biophys. Acta 1609, 80–86 (2003).
Gunnarson, E., Zelenina, M. & Aperia, A. Regulation of brain aquaporins. Neuroscience 129, 947–955 (2004).
King, L. S., Kozono, D. & Agre, P. From structure to disease: the evolving tale of aquaporin biology. Nature Rev. Mol. Cell Biol. 5, 687–698 (2004).
Badaut, J., Lasbennes, F., Magistretti, P. J. & Regli, L. Aquaporins in brain: distribution, physiology, and pathophysiology. J. Cereb Blood Flow Metab. 22, 367–378 (2002).
Francesca, B. & Rezzani, R. Aquaporin and blood brain barrier. Curr. Neuropharmacol. 8, 92–96 (2010).
Musa-Aziz, R., Chen, L. M., Pelletier, M. F. & Boron, W. F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl Acad. Sci. USA 106, 5406–5411 (2009).
Rigor, R. R., Hawkins, B. T. & Miller, D. S. Activation of PKC isoform beta(I) at the blood–brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J. Cereb Blood Flow Metab. 30, 1373–1383 (2010).
Raffa, R. B. & Tallarida, R. J. Effects on the visual system might contribute to some of the cognitive deficits of cancer chemotherapy-induced 'chemo-fog'. J. Clin. Pharm. Ther. 35, 249–255 (2010).
Hartz, A. M., Miller, D. S. & Bauer, B. Restoring blood–brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol. Pharmacol. 77, 715–723 (2010).
Loscher, W. & Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nature Rev. Neurosci. 6, 591–602 (2005).
Fletcher, J. I., Haber, M., Henderson, M. J. & Norris, M. D. ABC transporters in cancer: more than just drug efflux pumps. Nature Rev. Cancer 10, 147–156 (2010).
Schinkel, A. H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 36, 179–194 (1999).
Bauer, B., Hartz, A. M. & Miller, D. S. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood–brain barrier. Mol. Pharmacol. 71, 667–675 (2007).
Hartz, A. M., Bauer, B., Fricker, G. & Miller, D. S. Rapid regulation of P-glycoprotein at the blood–brain barrier by endothelin-1. Mol. Pharmacol. 66, 387–394 (2004).
Hartz, A. M., Bauer, B., Fricker, G. & Miller, D. S. Rapid modulation of P-glycoprotein-mediated transport at the blood–brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol. Pharmacol. 69, 462–470 (2006).
Bankstahl, J. P., Hoffmann, K., Bethmann, K. & Loscher, W. Glutamate is critically involved in seizure-induced overexpression of P-glycoprotein in the brain. Neuropharmacology 54, 1006–1016 (2008).
Bauer, B. et al. Seizure-induced up-regulation of P-glycoprotein at the blood–brain barrier through glutamate and cyclooxygenase-2 signalling. Mol. Pharmacol. 73, 1444–1453 (2008).
Pekcec, A. et al. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J. Pharmacol. Exp. Ther. 330, 939–947 (2009).
Zibell, G. et al. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology 56, 849–855 (2009).
Schlichtiger, J. et al. Celecoxib treatment restores pharmacosensitivity in a rat model of pharmacoresistant epilepsy. Br. J. Pharmacol. 160, 1062–1071 (2010).
Bauer, B. et al. In vivo activation of human pregnane X receptor tightens the blood–brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 70, 1212–1219 (2006).
Wang, X., Sykes, D. B. & Miller, D. S. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood–brain barrier. Mol. Pharmacol. 78, 376–383 (2010).
Wang, X., Hawkins, B. T. & Miller, D. S. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood–brain barrier. FASEB J. 25, 646–652 (2010).
Bauer, B., Hartz, A. M., Fricker, G. & Miller, D. S. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood–brain barrier. Mol. Pharmacol. 66, 413–419 (2004).
Dauchy, S. et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood–brain barrier. J. Neurochem. 107, 1518–1528 (2008).
Narang, V. S. et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood–brain barrier. Am. J. Physiol. Cell Physiol. 295, C440–450 (2008).
Ott, M., Fricker, G. & Bauer, B. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood–brain barrier: functional similarities between pig and human PXR. J. Pharmacol. Exp. Ther. 329, 141–149 (2009).
Miller, D. S., Bauer, B. & Hartz, A. M. Modulation of P-glycoprotein at the blood–brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 60, 196–209 (2008).
Hartz, A. M. & Bauer, B. Regulation of ABC transporters at the blood–brain barrier: new targets for CNS therapy. Mol. Interv. 10, 293–304 (2010).
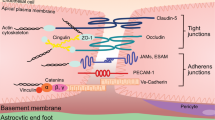
Hawkins, B. T. & Davis, T. P. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185 (2005).
Nitta, T. et al. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. Journal of Cell Biology 161, 653–660 (2003).
Saitou, M. et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 (2000).
Volterra, A. & Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Rev. Neurosci. 6, 626–640 (2005).
Wang, X. et al. Astrocytic Ca2+ signalling evoked by sensory stimulation in vivo. Nature Neurosci. 9, 816–823 (2006).
Ding, S. et al. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci. 27, 10674–10684 (2007).
Takata, N. & Hirase, H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS ONE 3, e2525 (2008).
Mulligan, S. J. & MacVicar, B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Zonta, M. et al. Neuron-to-astrocyte signalling is central to the dynamic control of brain microcirculation. Nature Neurosci. 6, 43–50 (2003).
Koehler, R. C., Roman, R. J. & Harder, D. R. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 30, 160–169 (2009).
Haydon, P. G. & Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031 (2006).
Gordon, G. R., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C. & MacVicar, B. A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749 (2008).
Simard, M., Arcuino, G., Takano, T., Liu, Q. S. & Nedergaard, M. Signalling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003).
Tam, S. J. & Watts, R. J. Connecting vascular and nervous system development: angiogenesis and the blood–brain barrier. Annu. Rev. Neurosci. 33, 379–408 (2010). A comprehensive, up-to-date review of parallels in angiogenesis and development of BBB properties.
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Stubbs, D. et al. Neurovascular congruence during cerebral cortical development. Cereb Cortex 19, (Suppl. 1) I32–I41 (2009).
Javaherian, A. & Kriegstein, A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex 19, (Suppl. 1) I70I77 (2009).
Risau, W. & Wolburg, H. Development of the blood–brain barrier. Trends Neurosci. 13, 174–178 (1990).
Harrigan, M. R. Angiogenic factors in the central nervous system. Neurosurgery 53, 639–660 (2003).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Stone, J. et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15, 4738–4747 (1995).
Jain, R. K. Molecular regulation of vessel maturation. Nature Med. 9, 685–693 (2003).
Greenberg, D. A. & Jin, K. From angiogenesis to neuropathology. Nature 438, 954–959 (2005).
Dziegielewska, K. M. et al. Plasma proteins in fetal sheep brain: blood–brain barrier and intracerebral distribution. J. Physiol. 318, 239–250 (1981).
Dziegielewska, K. M. et al. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J. Physiol. 292, 207–231 (1979).
Evans, C. A., Reynolds, J. M., Reynolds, M. L., Saunders, N. R. & Segal, M. B. The development of a blood–brain barrier mechanism in foetal sheep. J. Physiol. 238, 371–386 (1974).
Mollgard, K. & Saunders, N. R. The development of the human blood–brain and blood–CSF barriers. Neuropathol. Appl. Neurobiol. 12, 337–358 (1986).
Johansson, P. A., Dziegielewska, K. M., Liddelow, S. A. & Saunders, N. R. The blood–CSF barrier explained: when development is not immaturity. Bioessays 30, 237–248 (2008).
Ek, C. J., Dziegielewska, K. M., Stolp, H. & Saunders, N. R. Functional effectiveness of the blood–brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 496, 13–26 (2006).
Gazzin, S. et al. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood–brain interfaces. J. Comp. Neurol. 510, 497–507 (2008).
Kalabis, G. M., Petropoulos, S., Gibb, W. & Matthews, S. G. Breast cancer resistance protein (Bcrp1/Abcg2) in mouse placenta and yolk sac: ontogeny and its regulation by progesterone. Placenta 28, 1073–1081 (2007).
Ek, C. J. et al. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol. Lett. 197, 51–59 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010). A comprehensive study of the involvement of pericytes in BBB development.
Daneman, R. et al. The mouse blood–brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 5, e13741 (2010). A large-scale screen of gene expression in adult and postnatal cerebral endothelial cells compared with liver and lung endothelial cells.
Wosik, K. et al. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J. Neurosci. 27, 9032–9042 (2007).
Liddelow, S. A. et al. Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica. Eur. J. Neurosci. 29, 253–266 (2009).
Liddelow, S. A. et al. Modification of protein transfer across blood/cerebrospinal fluid barrier in response to altered plasma protein composition during development. Eur. J. Neurosci. 33, 391–400 (2010).
Krasney, J. A. A neurogenic basis for acute altitude illness. Med. Sci. Sports Exerc. 26, 195–208 (1994).
Severinghaus, J. W. Hypothetical roles of angiogenesis, osmotic swelling, and ischemia in high-altitude cerebral edema. J. Appl. Physiol. 79, 375–379 (1995).
Wilson, M. H., Newman, S. & Imray, C. H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 8, 175–191 (2009).
Laursen, H. Cerebral vessels and glial cells in liver disease. A morphometric and electron microscopic investigation. Acta Neurol. Scand. 65, 381–412 (1982).
Martinez, A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol. 11, 82–86 (1968).
Tripathi, A. K., Sha, W., Shulaev, V., Stins, M. F. & Sullivan, D. J. Jr. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood 114, 4243–4252 (2009).
Tripathi, A. K., Sullivan, D. J. & Stins, M. F. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-kappaB. Infect. Immun. 74, 3262–3270 (2006).
Tripathi, A. K., Sullivan, D. J. & Stins, M. F. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood–brain barrier endothelial cell monolayers. J. Infect. Dis. 195, 942–950 (2007).
Engelhardt, B. & Kappos, L. Natalizumab: targeting alpha4-integrins in multiple sclerosis. Neurodegener. Dis. 5, 16–22 (2008).
Spencer, B. J. & Verma, I. M. Targeted delivery of proteins across the blood–brain barrier. Proc. Natl Acad. Sci. USA 104, 7594–7599 (2007).
Hoffman, A. S. The origins and evolution of 'controlled' drug delivery systems. J. Control Release 132, 153–163 (2008).
Pardridge, W. M. Biopharmaceutical drug targeting to the brain. J. Drug Target 18, 157–167 (2010). A review of methods for delivering large molecules to the brain.
Vastag, B. Biotechnology: Crossing the barrier. Nature 466, 916–918 (2010).
Farkas, E., De Jong, G. I., de Vos, R. A., Jansen Steur, E. N. & Luiten, P. G. Pathological features of cerebral cortical capillaries are doubled in Alzheimer's disease and Parkinson's disease. Acta Neuropathol. 100, 395–402 (2000).
Levy, E. et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248, 1124–1126 (1990).
Herzig, M. C. et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nature Neurosci. 7, 954–960 (2004).
Premkumar, D. R., Cohen, D. L., Hedera, P., Friedland, R. P. & Kalaria, R. N. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am. J. Pathol. 148, 2083–2095 (1996).
Westerlund, M. et al. Association of a polymorphism in the ABCB1 gene with Parkinson's disease. Parkinsonism Relat Disord. 15, 422–424 (2009).
Weber, Y. G. et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J. Clin. Invest. 118, 2157–2168 (2008).
Suls, A. et al. Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1. Brain 131, 1831–1844 (2008).
Newmeyer, A., Cecil, K. M., Schapiro, M., Clark, J. F. & Degrauw, T. J. Incidence of brain creatine transporter deficiency in males with developmental delay referred for brain magnetic resonance imaging. J. Dev. Behav. Pediatr. 26, 276–282 (2005).
Klepper, J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia 49 (Suppl. 8) 46–49 (2008).
Kim, W. S., Weickert, C. S. & Garner, B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 104, 1145–1166 (2008).
Drozdzik, M. et al. Polymorphism in the P-glycoprotein drug transporter MDR1 gene: a possible link between environmental and genetic factors in Parkinson's disease. Pharmacogenetics 13, 259–263 (2003).
Dean, M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 400, 409–429 (2005).
Blanz, J. et al. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J. Neurosci. 27, 6581–6589 (2007).
Fabene, P. F. et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nature Med. 14, 1377–1383 (2008).
Kreutzberg, G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
Appel, S. H., Beers, D. R. & Henkel, J. S. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 31, 7–17 (2010).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nature Methods 5, 877–879 (2008).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Seeley, E. H. & Caprioli, R. M. Molecular imaging of proteins in tissues by mass spectrometry. Proc. Natl Acad. Sci. USA 105, 18126–18131 (2008).
Todman, M. G., Han, S. K. & Herbison, A. E. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 132, 703–712 (2005).
Turney, S. G. & Lichtman, J. W. Imaging fluorescent mice in vivo using confocal microscopy. Methods Cell Biol. 89, 309–327 (2008).
Zhuo, L. et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev. Biol. 187, 36–42 (1997).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nature Med. 7, 1356–1361 (2001).
Miesenbock, G. The optogenetic catechism. Science 326, 395–399 (2009).
Iqbal, U. et al. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br. J. Cancer 103, 1606–1616 (2010).
Elsinga, P. H., Hendrikse, N. H., Bart, J., van Waarde, A. & Vaalburg, W. Positron emission tomography studies on binding of central nervous system drugs and P-glycoprotein function in the rodent brain. Mol. Imaging Biol. 7, 37–44 (2005).
Weinstein, J. S. et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb Blood Flow Metab. 30, 15–35 (2010). This shows the current status of research into iron oxide nanoparticles, which are becoming increasingly important in dynamic MRI of brain tumours and in assessing inflammation in a variety of neurologic diseases.
Muldoon, L. L. et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J. Clin. Oncol. 25, 2295–2305 (2007). This paper demonstrated the important finding that most chemotherapy crosses the blood–tumour barrier to some degree but not nearly as well as systemic tissues, even in highly malignant tumours.
Angelov, L. et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J. Clin. Oncol. 27, 3503–3509 (2009).
Eliceiri, B. P., Gonzalez, A. M. & Baird, A. Zebrafish model of the blood–brain barrier: morphological and permeability studies. Methods Mol. Biol. 686, 371–378 (2011). An important new model for in vivo BBB studies, especially in development.
Hartz, A. M. & Bauer, B. ABC Transporters in the CNS — an inventory. Curr. Pharm. Biotechnol. 12, 423–440 (2011).
Saunders, N. R., Ek, C. J., Habgood, M. D. & Dziegielewska, K. M. Barriers in the brain: a renaissance? Trends Neurosci. 31, 279–286 (2008).
Bates, K. A. et al. Clearance mechanisms of Alzheimer's amyloid-beta peptide: implications for therapeutic design and diagnostic tests. Mol. Psychiatry 16, 16 (2008).
Vogelgesang, S. et al. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 12, 535–541 (2002).
Cirrito, J. R. et al. P-glycoprotein deficiency at the blood–brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290 (2005).
Xiong, H. et al. ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood–brain barrier for Abeta(1–40) peptides. J. Neurosci. 29, 5463–5475 (2009).
Lu, A. et al. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp. Neurol. 210, 549–559 (2008).
Rosenberg, G. A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 8, 205–216 (2009).
Wang, X., Rosell, A. & Lo, E. H. Targeting extracellular matrix proteolysis for hemorrhagic complications of tPA stroke therapy. CNS Neurol. Disord. Drug Targets. 7, 235–242 (2008).
Xue, M. & Yong, V. W. Matrix metalloproteinases in intracerebral hemorrhage. Neurol. Res. 30, 775–782 (2008).
Argaw, A. T., Gurfein, B. T., Zhang, Y., Zameer, A. & John, G. R. VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc. Natl Acad. Sci. USA. 106(6):1977–1982 (2009).
Guzeloglu-Kayisli, O. et al. KRIT1/cerebral cavernous malformation 1 protein localizes to vascular endothelium, astrocytes, and pyramidal cells of the adult human cerebral cortex. Neurosurgery 54, 943–949; discussion 949 (2004).
Pagenstecher, A., Stahl, S., Sure, U. & Felbor, U. A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum. Mol. Genet. 18, 911–918 (2009).
Candelario-Jalil, E., Yang, Y. & Rosenberg, G. A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158, 983–994 (2009).
Gidday, J. M. et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood–brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am. J. Physiol. Heart Circ. Physiol. 289, H558–H568 (2005).
Strbian, D. et al. The blood–brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 153, 175–181 (2008).
Simard, J. M. et al. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nature Med. 12, 433–440 (2006).
Hughes, J. R. One of the hottest topics in epileptology: ABC proteins. Their inhibition may be the future for patients with intractable seizures. Neurol. Res. 30, 920–925 (2008).
Mignot, C. et al. Alexander disease: putative mechanisms of an astrocytic encephalopathy. Cell. Mol. Life Sci. 61, 369–385 (2004).
Chaudhuri, A., Yang, B., Gendelman, H. E., Persidsky, Y. & Kanmogne, G. D. STAT1 signalling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood–brain barrier. Blood 111, 2062–2072 (2008).
Huang, W., Eum, S. Y., Andras, I. E., Hennig, B. & Toborek, M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 23, 1596–1606 (2009).
Yamamoto, M. et al. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 172, 521–533 (2008).
de Lagerie, S. B. et al. MDR1A (ABCB1)-deficient CF-1 mutant mice are susceptible to cerebral malaria induced by Plasmodium berghei ANKA. J. Parasitol. 94, 1139–1142 (2008).
Unkmeir, A. et al. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol 46, 933–946 (2002).
Wang, L. & Lin, M. A novel cell wall-anchored peptidoglycan hydrolase (autolysin), IspC, essential for Listeria monocytogenes virulence: genetic and proteomic analysis. Microbiology 154, 1900–1913 (2008).
Banks, W. A. Physiology and pathology of the blood–brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J. Neurovirol. 5, 538–555 (1999).
Haorah, J. et al. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J. Neurochem. 101, 566–576 (2007).
Man, S., Ubogu, E. E. & Ransohoff, R. M. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 17, 243–250 (2007).
Suidan, G. L., McDole, J. R., Chen, Y., Pirko, I. & Johnson, A. J. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PLoS ONE 3, e3037 (2008).
Verkman, A. S. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev. Mol. Med. 10, e13 (2008).
Yang, B., Zador, Z. & Verkman, A. S. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J. Biol. Chem. 283, 15280–15286 (2008).
Blecharz, K. G., Drenckhahn, D. & Forster, C. Y. Glucocorticoids increase VE-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cEND cells. J. Cereb Blood Flow Metab. 28, 1139–1149 (2008).
Felinski, E. A., Cox, A. E., Phillips, B. E. & Antonetti, D. A. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp. Eye Res. 86, 867–878 (2008).
Harke, N., Leers, J., Kietz, S., Drenckhahn, D. & Forster, C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol. Cell Endocrinol. 295, 39–47 (2008).
Reijerkerk, A. et al. Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J. Immunol. 181, 3567–3574 (2008).
Kebir, H. et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nature Med. 13, 1173–1175 (2007).
Morgan, L. et al. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147, 664–673 (2007).
Goodin, D. S., Cohen, B. A., O'Connor, P., Kappos, L. & Stevens, J. C. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 71, 766–773 (2008).
Lutterotti, A. & Martin, R. Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol. 7, 538–547 (2008).
Martin, C. et al. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev. Biol. 297, 402–416 (2006).
Brooks, T. A. et al. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 1120, 172–182 (2006).
Foroutan, S., Brillault, J., Forbush, B. & O'Donnell, M. E. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl− cotransporter. Am. J. Physiol. Cell Physiol. 289, C1492–C1501 (2005).
Lam, T. I., Anderson, S. E., Glaser, N. & O'Donnell, M. E. Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes 54, 510–516 (2005).
Lam, T. I. & O'Donnell, M. E. BBB Na/H exchange: plasma membrane distribution of NHE1 and NHE2 isoforms and stimulation by arginine vasopressin. FASEB J. 22, 734.3 (2008).
O'Donnell, M. E., Duong, V., Suvatne, J., Foroutan, S. & Johnson, D. M. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransporter activity is V1 receptor and [Ca] dependent. Am. J. Physiol. Cell Physiol. 289, C283–292 (2005).
O'Donnell, M. E., Lam, T. I., Tran, L. & Anderson, S. E. The role of the blood–brain barrier Na-K-2Cl cotransporter in stroke. Adv. Exp. Med. Biol. 559, 67–75 (2004).
Yuen, N., Anderson, S. E., Glaser, N., Tancredi, D. J. & O'Donnell, M. E. Cerebral blood flow and cerebral edema in rats with diabetic ketoacidosis. Diabetes 57, 2588–2594 (2008).
Banks, W. A. The blood–brain barrier as a cause of obesity. Curr. Pharm. Des. 14, 1606–1614 (2008).
Jequier, E. Leptin signalling, adiposity, and energy balance. Ann. NY Acad. Sci. 967, 379–388 (2002).
Pan, W. et al. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149, 2798–2806 (2008).
Luder, A. S., Tanner, S. M., de la Chapelle, A. & Walter, J. H. Amnionless (AMN) mutations in Imerslund-Grasbeck syndrome may be associated with disturbed vitamin B(12) transport into the CNS. J. Inherit Metab. Dis. 7, 7 (2008).
Gordon, N. Canavan disease: a review of recent developments. Eur. J. Paediatr. Neurol. 5, 65–69 (2001).
Urayama, A., Grubb, J. H., Sly, W. S. & Banks, W. A. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood–brain barrier. Proc. Natl Acad. Sci. USA 101, 12658–12663 (2004).
Uhr, M. et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron 57, 203–209 (2008).
Tanigami, H. et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 131, 437–449 (2005).
Sugimoto, H., Koehler, R. C., Wilson, D. A., Brusilow, S. W. & Traystman, R. J. Methionine sulfoximine, a glutamine synthetase inhibitor, attenuates increased extracellular potassium activity during acute hyperammonemia. J. Cereb Blood Flow Metab. 17, 44–49 (1997).
Strauss, G. I., Knudsen, G. M., Kondrup, J., Moller, K. & Larsen, F. S. Cerebral metabolism of ammonia and amino acids in patients with fulminant hepatic failure. Gastroenterology 121, 1109–1119 (2001).
Acknowledgements
The meeting on which this report was based was partially funded by an R13 grant from the US National Institutes of Health (Grant 5 R13 CA086959-10). We would like to thank all of the people who attended the Engaging Neuroscience to Advance Brain Barriers Translational Research meeting (March 19–21, 2009), Gleneden Beach, Oregon, USA. Special thanks to Lester Drewes, Martha O'Donnell, Leslie Muldoon and Aliana Kim who were instrumental in the development of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (box)
Co-Chairs and Members of the Five Committees (PDF 164 kb)
Supplementary information S2 (box)
“Engaging Neuroscience to Advance Brain Barriers Translational Research” (PDF 171 kb)
Supplementary information S3 (box)
Molecular Physiology of the Brain and Brain Barriers Working Group Report (PDF 6070 kb)
Related links
Glossary
- Abluminal
-
Facing the neural cells or brain.
- Basal lamina
-
A thin, continuous layer of extracellular matrix surrounding the brain endothelial cells and pericytes.
- Blood–cerebrospinal fluid (CSF) barrier
-
The blood–CSF barrier is at the choroid plexus epithelial cells, which are joined together by tight junctions. The capillaries in the choroid plexus differ from those of the blood–brain barrier in that there is free movement of molecules between endothelial cells via fenestrations and intercellular gaps.
- Blood–labyrinth barrier
-
The cochlea is a structure of the inner ear involved in sound transduction and is vascularized by a dense set of capillaries that are essential for delivering the nutrients and ions necessary for producing the fluids (endolymph and perilymph) present in the cochlea. These capillaries are lined with endothelial cells that are joined by tight junctions and physiologically form the blood–labyrinth barrier that is essential for sensitive auditory function.
- Blood–nerve barrier
-
The endothelial lining of blood vessels in peripheral nerves is formed by continuous, non-fenestrated endothelia in which individual cells are linked by tight junctions, rendering them impermeable to intravascular macromolecules. This blood–nerve barrier, and a similar mechanism in the innermost perineurial sheath, isolate the endoneurial interstitium, in much the same way as the blood–brain barrier. Other factors, such as the absence of lymphatics, are also analogous to the central nervous system.
- Blood–retinal barrier
-
The blood–retinal barrier has two components: the retinal vascular endothelium and the retinal pigment epithelium. The retinal vascular endothelium is non-fenestrated and has anatomical properties similar to those of cerebral vascular endothelium. The retinal pigment epithelium consists of a layer of epithelial cells, joined by tight junctions, that forms a barrier between the neuroretina and the choroid.
- Ependyma
-
A thin cellular layer lining the ventricular system of the brain. The cells of the ependyma are called ependymal cells and are a type of glia. They are linked by gap junctions, which do not provide an impediment to diffusion of molecules, even against large proteins between cerebrospinal fluid and brain interstitial fluid.
- Luminal
-
Facing the capillary lumen.
- Neuro–angiogenic coupling
-
The coupling of the development of neurons (neurogenesis) with new blood vessel formation (angiogenesis and vasculogenesis).
- Neuroependyma
-
(Also known as neuroepithelium or ventricular zone.) A deep pseudostratified layer of cells lining the embryonic ventricular system that proliferate into radial glial cells and neurons in the embryo, and into glial cells later in development. The cells of the neuroependyma are linked by strap junctions, which limit intercellular movement of molecules — particularly proteins — from cerebrospinal fluid to brain interstitial space in the embryo. By adulthood these cells have transformed to the layer of thin generally non-dividing ependymal cells lining the ventricular system of the mature brain.
- Neuro–haemodynamic coupling
-
The coupling of neuronal firing and synaptic activity with haemodynamic changes (for example, blood volume and blood flow).
- Neuro–metabolic coupling
-
The coupling of neural activity, an energy consuming process, with the energy producing metabolic processes to maintain cellular homeostasis.
- Neuro–trophic coupling
-
The coupling of neuronal production of activity-dependent signals such as growth factors (for example, brain-derived neurotrophic factor (BDNF)) with control of neurogenesis.
- Paracellular
-
Paracellular is used here to refer to the transfer of substances between cells of an endothelium or epithelium. It is in contrast to 'transcellular transport', in which the substances are transported through the cell.
- Tripartite synapse
-
A tripartite (three-part) synapse consists of a presynapse, a postsynapse and a glial cell functioning as a single unit.
- Xenobiotic-sensing nuclear receptor
-
A xenobiotic-activated transcription factor that controls the expression of proteins involved in xenobiotic metabolism and efflux transport.
Rights and permissions
About this article
Cite this article
Neuwelt, E., Bauer, B., Fahlke, C. et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12, 169–182 (2011). https://doi.org/10.1038/nrn2995
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2995
This article is cited by
-
Subcellular analysis of blood-brain barrier function by micro-impalement of vessels in acute brain slices
Nature Communications (2023)
-
Vaccines for prion diseases: a realistic goal?
Cell and Tissue Research (2023)
-
CD98hc is a target for brain delivery of biotherapeutics
Nature Communications (2023)
-
NLRP3 inflammasome-mediated choroid plexus hypersecretion contributes to hydrocephalus after intraventricular hemorrhage via phosphorylated NKCC1 channels
Journal of Neuroinflammation (2022)
-
Caveolin-1 accelerates hypoxia-induced endothelial dysfunction in high-altitude cerebral edema
Cell Communication and Signaling (2022)