Key Points
-
There is evidence for the occurrence of programmed cell death (PCD) in several neurodegenerative disorders. Most of the evidence comes from studies in animal models of conditions such as Parkinson's and Huntington's diseases (PD and HD, respectively), and amyotrophic lateral sclerosis (ALS).
-
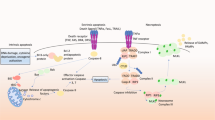
There are two basic pathways of PCD: the mitochondrial (intrinsic) and the death receptor (extrinsic) pathways. Both pathways require the involvement of proteases known as caspases, the activity of which is regulated by different proteins of the so-called Bcl2 family.
-
PCD has been proposed to take place in the brains of people with PD, although the best evidence in favour of this idea has come from studies of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of parkinsonism. Here, MPTP administration is accompanied by the activation of caspases. Moreover, drugs that interfere with early stages of PCD have been successful at preventing neurodegeneration in this model.
-
PCD has also been documented to take place in association with ALS, mostly in transgenic models of the disease. In this case, interfering with PCD delays neuronal death and prolongs survival. However, as death eventually ensues, it seems that targeting PCD in ALS can slow the death process, but cannot stop it altogether.
-
The data in relation to PCD in HD is still incomplete, but there are reasons to believe that caspase activation might participate in the neuronal death that accompanies this disease.
-
The PCD machinery might be a good target for the development of drugs against these neurodegenerative disorders. However, PCD is only one of the processes that contributes to the pathological changes, making it necessary to think in terms of attacking the problem from other positions. In addition, therapeutic efforts should take into account that caspase activation might be a late process in the PCD cascade, a fact that might limit their usefulness as drug targets.
Abstract
Molecular pathways of programmed cell death (PCD) are activated in various neurodegenerative disorders including Parkinson's disease, amyotrophic lateral sclerosis and Huntington's disease. In these diseases, PCD might be pathogenic, and targeting it might mitigate neurodegeneration. To identify potential neuroprotective targets within the PCD machinery, the expression and activity of some of its components have been altered by genetic or pharmacological means in experimental models of neurodegenerative diseases. The results of these studies have provided leads for the development of neuroprotective strategies for these progressive, disabling and often fatal disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clarke, P. G. H. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. 181, 195–213 (1990).
Clarke, P. G. H. Cell death and diseases of the nervous system (eds Koliatsos, V. E. & Ratan, R. R.) 3–28 (Humana Press, New Jersey, 1999).
Sperandio, S., de Belle, I. & Bredesen, D. E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl Acad. Sci. USA 97, 14376–14381 (2000).
Kane, D. J., Örd, T., Anton, R. & Bredesen, D. E. Expression of bcl-2 inhibits necrotic neural cell death. J. Neurosci. Res. 40, 269–275 (1995).
Sloviter, R. S. Apoptosis: a guide for the perplexed. Trends Pharmacol. Sci. 23, 19–24 (2002).
Kingsbury, A. E., Mardsen, C. D. & Foster, O. J. DNA fragmentation in human substantia nigra: apoptosis or perimortem effect? Mov. Disord. 13, 877–884 (1998).
Marx, J. New leads on the 'how' of Alzheimer's. Science 293, 2192–2194 (2001).
Graham, S. H. & Chen, J. Programmed cell death in cerebral ischemia. J. Cereb. Blood Flow Metab. 21, 99–109 (2001).
Przedborski, S., Vila, M. & Jackson-Lewis, V. Neurodegeneration: what is it and where are we? J. Clin. Invest. 111, 3–10 (2003).
Muzio, M., Stockwell, B. R., Stennicke, H. R., Salvesen, G. S. & Dixit, V. M. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273, 2926–2930 (1998).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Gross, A., McDonnell, J. M. & Korsmeyer, S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183 (2002).
Marsden, V. S. et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 634–637 (2002).
Wyss-Coray, T. & Mucke, L. Inflammation in neurodegenerative disease — a double-edged sword. Neuron 35, 419–432 (2002).
Slee, E. A. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspase-2,-3,-6,-7,-8, and-10 in a caspase-9-dependent manner. J. Cell Biol. 144, 281–292 (1999).
Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15, 269–290 (1999).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 (2000).
Chai, J. et al. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406, 855–862 (2000).
Srinivasula, S. M. et al. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol. Chem. 275, 36152–36157 (2000).
Susin, S. A. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 (1999).
Li, L. Y., Luo, X. & Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99 (2001).
Wang, X., Yang, C., Chai, J., Shi, Y. & Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science 298, 1587–1592 (2002).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Morishima, N., Nakanishi, K., Takenouchi, H., Shibata, T. & Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294 (2002).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 2003 Mar 6 (DOI 10.1126/science.1081208).
Fahn, S. & Przedborski, S. Merritt's neurology (ed. Rowland, L. P) 679–693 (Lippincott, Williams & Wilkins, New York, 2000).
Mochizuki, H., Goto, K., Mori, H. & Mizuno, Y. Histochemical detection of apoptosis in Parkinson's disease. J. Neurol. Sci. 137, 120–123 (1996).
Anglade, P. et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol. Histopathol. 12, 25–31 (1997).
Tatton, N. A., Maclean-Fraser, A., Tatton, W. G., Perl, D. P. & Olanow, C. W. A fluorescent double-labeling method to detect and confirm apoptotic nuclei in Parkinson's disease. Ann. Neurol. 44, S142–S148 (1998).
Tompkins, M. M., Basgall, E. J., Zamrini, E. & Hill, W. D. Apoptotic-like changes in Lewy-body-associated disorders and normal aging in substantia nigral neurons. Am. J. Pathol. 150, 119–131 (1997).
Kosel, S., Egensperger, R., Von Eitzen, U., Mehraein, P. & Graeber, M. B. On the question of apoptosis in the parkinsonian substantia nigra. Acta Neuropathol. (Berl.) 93, 105–108 (1997).
Banati, R. B., Daniel, S. E. & Blunt, S. B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov. Disord. 13, 221–227 (1998).
Wullner, U. et al. Cell death and apoptosis regulating proteins in Parkinson's disease — a cautionary note. Acta Neuropathol. (Berl.) 97, 408–412 (1999).
Hartmann, A. et al. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson's disease? J. Neurochem. 76, 1785–1793 (2001).
Tatton, N. A. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp. Neurol. 166, 29–43 (2000).
Hartmann, A. et al. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc. Natl Acad. Sci. USA 97, 2875–2880 (2000).
Hartmann, A. et al. Increased expression and redistribution of the antiapoptotic molecule Bcl-xL in Parkinson's disease. Neurobiol. Dis. 10, 28–32 (2002).
Hartmann, A. et al. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease, but pathway inhibition results in neuronal necrosis. J. Neurosci. 21, 2247–2255 (2001).
Viswanath, V. et al. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J. Neurosci. 21, 9519–9528 (2001).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 3, 1301–1306 (2000).
Hoglinger, G. U. et al. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 84, 1–12 (2003).
Masliah, E. et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 (2000).
van der Putten, H. et al. Neuropathology in mice expressing human α-synuclein. J. Neurosci. 20, 6021–6029 (2000).
Feany, M. B. & Bender, W. W. A Drosophila model of Parkinson's disease. Nature 404, 394–398 (2000).
Matsuoka, Y. et al. Lack of nigral pathology in transgenic mice expressing human α-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol. Dis. 8, 535–539 (2001).
Lee, M. K. et al. Human α-synuclein-harboring familial Parkinson's disease-linked Ala-53-Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice PG-8968-73. Proc. Natl Acad. Sci. USA 99, 8968–73 (2002).
Jackson-Lewis, V., Jakowec, M., Burke, R. E. & Przedborski, S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257–269 (1995).
Tatton, N. A. & Kish, S. J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77, 1037–1048 (1997).
Vila, M. et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 2837–2842 (2001). Direct evidence indicating that the pro-PCD protein Bax is essential in MPTP-induced neurodegeneration.
Yang, L. et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J. Neurosci. 18, 8145–8152 (1998).
Offen, D. et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Proc. Natl Acad. Sci. USA 95, 5789–5794 (1998).
Thornborrow, E. C., Patel, S., Mastropietro, A. E., Schwartzfarb, E. M. & Manfredi, J. J. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene 21, 990–999 (2002).
Mandir, A. S. et al. A novel in vivo post-translational modification of p53 by PARP-1 in MPTP-induced parkinsonism. J. Neurochem. 83, 186–192 (2002).
Mandir, A. S. et al. Poly (ADP-ribose) polymerase activation mediates MPTP-induced parkinsonism. Proc. Natl Acad. Sci. USA 96, 5774–5779 (1999).
Duan, W. et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 52, 597–606 (2002). This study demonstrates that intraperitoneal injections of two p53 inhibitors attenuates Bax upregulation, improves motor function, reduces damage to nigrostriatal DA neurons and reduces depletion of DA and its metabolites in MPTP-treated mice.
Trimmer, P. A., Smith, T. S., Jung, A. B. & Bennett, J. P. Jr. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5, 233–239 (1996).
Saporito, M. S., Thomas, B. A. & Scott, R. W. MPTP activates c-Jun NH2-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J. Neurochem. 75, 1200–1208 (2000).
Xia, X. G. et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 10433–10438 (2001). Direct evidence of the involvement of the JNK/Jun pathway in MPTP-induced apoptotic cell death.
Saporito, M. S., Brown, E. M., Miller, M. S. & Carswell, S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J. Pharmacol. Exp. Ther. 288, 421–427 (1999).
Tournier, C. et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288, 870–874 (2000).
Lei, K. et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun N-terminal kinase. Mol. Cell Biol. 22, 4929–4942 (2002).
Mochizuki, H. et al. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 10918–10923 (2001). Using viral vectors to deliver an Apaf1 inhibitor, this paper shows the instrumental role of the mitochondrial PCD pathway in MPTP-mediated neurodegeneration.
Eberhardt, O. et al. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Neurosci. 20, 9126–9134 (2000). Evidence indicating that adenoviral gene transfer of XIAP, a protein caspase inhibitor, can prevent cell death of DA neurons induced by MPTP, but it does not protect against MPTP-induced reduction of striatal cathecolamine concentrations.
Bilsland, J. et al. Caspase inhibitors attenuate 1-methyl-4-phenylpyridinium toxicity in primary cultures of mesencephalic dopaminergic neurons. J. Neurosci. 22, 2637–2649 (2002).
Raff, M. C., Whitmore, A. V. & Finn, J. T. Axonal self-destruction and neurodegeneration. Science 296, 868–871 (2002).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 6, 797–801 (2000). Demonstration that inhibition of caspase activation by a second-generation tetracycline derivative delays disease progression and mortality in a transgenic mouse model of HD.
Du, Y. et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 14669–14674 (2001).
Wu, D. C. et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22, 1763–1771 (2002).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nature Genet. 18, 107–108 (1998).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Vila, M. et al. α-Synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J. Neurochem. 74, 721–729 (2000).
Przedborski, S. et al. Oxidative post-translational modifications of α-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J. Neurochem. 76, 637–640 (2001).
Dauer, W. et al. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl Acad. Sci. USA 99, 14524–14529 (2002).
El-Agnaf, O. M. et al. Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS Lett. 440, 71–75 (1998).
Hashimoto, M., Takeda, A., Hsu, L. J., Takenouchi, T. & Masliah, E. Role of cytochrome c as a stimulator of α-synuclein aggregation in Lewy body disease. J. Biol. Chem. 274, 28849–28852 (1999).
Kakimura, J. et al. Release and aggregation of cytochrome c and α-synuclein are inhibited by the antiparkinsonian drugs, talipexole and pramipexole. Eur. J. Pharmacol. 417, 59–67 (2001).
Riederer, P. et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 52, 515–520 (1989).
Nicole, A., Santiard-Baron, D. & Ceballos-Picot, I. Direct evidence for glutathione as mediator of apoptosis in neuronal cells. Biomed. Pharmacother. 52, 349–355 (1998).
Rowland, L. P. Merritt's textbook of neurology (ed. Rowland, L. P.) 742–749 (Lippincott, Williams & Wilkins, Philadelphia, 1995).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P. C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Bruijn, L. I. et al. ALS-linked SOD1 mutant G85R mediated damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Nagai, M. et al. Rats expressing human cytosolic copper–zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J. Neurosci. 21, 9246–9254 (2001).
Subramaniam, J. R. et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nature Neurosci. 5, 301–307 (2002).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 13, 43–47 (1996).
Brown, R. H. Jr. Superoxide dismutase in familial amyotrophic lateral sclerosis: models for gain of function. Curr. Opin. Neurobiol. 5, 841–846 (1995).
Rabizadeh, S. et al. Mutations associated with amyotrophic lateral sclerosis convert superoxide dismutase from an antiapoptotic gene to a proapoptotic gene: studies in yeast and neural cells. Proc. Natl Acad. Sci. USA 92, 3024–3028 (1995).
Ghadge, G. D. et al. Mutant superoxide dismutase-1-linked familial amyotrophic lateral sclerosis: molecular mechanisms of neuronal death and protection. J. Neurosci. 17, 8756–8766 (1997).
Mena, M. A. et al. Effects of wild-type and mutated copper/zinc superoxide dismutase on neuronal survival and L-DOPA-induced toxicity in postnatal midbrain culture. J. Neurochem. 69, 21–33 (1997).
Kostic, V., Jackson-Lewis, V., De Bilbao, F., Dubois-Dauphin, M. & Przedborski, S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science 277, 559–562 (1997). Demonstration that overexpression of Bcl2 mitigates neurodegeneration and prolongs survival in a transgenic mouse model of familial ALS.
Li, M. et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288, 335–339 (2000). Demonstration that the irreversible broad-caspase inhibitor zVAD-fmk attenuates mutant SOD1-mediated cell death in a transgenic mouse model of ALS, resulting in a delayed disease onset and mortality.
Guegan, C. & Przedborski, S. Programmed cell death in amyotrophic lateral sclerosis. J. Clin. Invest. 111, 153–161 (2003).
Cleveland, D. W. & Rothstein, J. D. From Charcot to Lou Gehrig deciphering selective motor neuron death in ALS. Nature Rev. Neurosci. 2, 806–819 (2001).
Hirano, A. Aspects of the ultrastructure of amyotrophic lateral sclerosis. Adv. Neurol. 36, 75–88 (1982).
Martin, L. J. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 58, 459–471 (1999).
Vukosavic, S. et al. Delaying caspase activation by Bcl-2: a clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 20, 9119–9125 (2000).
Pasinelli, P., Houseweart, M. K., Brown, R. H. Jr & Cleveland, D. W. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 97, 13901–13906 (2000).
Dal Canto, M. C. & Gurney, M. E. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 145, 1271–1279 (1994).
Yoshiyama, Y., Yamada, T., Asanuma, K. & Asahi, T. Apoptosis related antigen, LeY and nick-end labeling are positive in spinal motor neurons in amyotrophic lateral sclerosis. Acta Neuropathol. (Berl.) 88, 207–211 (1994).
Ekegren, T., Grundstrom, E., Lindholm, D. & Aquilonius, S. M. Upregulation of Bax protein and increased DNA degradation in ALS spinal cord motor neurons. Acta Neurol. Scand. 100, 317–321 (1999).
Migheli, A., Cavalla, P., Marino, S. & Schiffer, D. A study of apoptosis in normal and pathologic nervous tissue after in situ end-labeling of DNA strand breaks. J. Neuropathol. Exp. Neurol. 53, 606–616 (1994).
Migheli, A. et al. Lack of apoptosis in mice with ALS. Nature Med. 5, 966–967 (1999).
He, B. P. & Strong, M. J. Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. A comparative study of ALS and chronic aluminium chloride neurotoxicity in New Zealand white rabbits. Neuropathol. Appl. Neurobiol. 26, 150–160 (2000).
Pedersen, W. A., Luo, H., Kruman, I., Kasarskis, E. & Mattson, M. P. The prostate apoptosis response-4 protein participates in motor neuron degeneration in amyotrophic lateral sclerosis. FASEB J. 14, 913–924 (2000).
Mu, X., He, J., Anderson, D. W., Trojanowski, J. Q. & Springer, J. E. Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann. Neurol. 40, 379–386 (1996).
Vukosavic, S., Dubois-Dauphin, M., Romero, N. & Przedborski, S. Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 73, 2460–2468 (1999).
Troost, D., Aten, J., Morsink, F. & De Jong, J. M. B. V. Apoptosis in amyotrophic lateral sclerosis is not restricted to motor neurons. Bcl-2 expression is increased in unaffected post- central gyrus. Neuropathol. Appl. Neurobiol. 21, 498–504 (1995).
Gonzalez de Aguilar, J. L. et al. Alteration of the Bcl-x/Bax ratio in a transgenic mouse model of amyotrophic lateral sclerosis: evidence for the implication of the p53 signaling pathway. Neurobiol. Dis. 7, 406–415 (2000).
Guegan, C., Vila, M., Rosoklija, G., Hays, A. P. & Przedborski, S. Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J. Neurosci. 21, 6569–6576 (2001).
Martin, L. J. p53 is abnormally elevated and active in the CNS of patients with amyotrophic lateral sclerosis. Neurobiol. Dis. 7, 613–622 (2000).
Prudlo, J. et al. Motor neuron cell death in a mouse model of FALS is not mediated by the p53 cell survival regulator. Brain Res. 879, 183–187 (2000).
Kuntz, C., Kinoshita, Y., Beal, M. F., Donehower, L. A. & Morrison, R. S. Absence of p53: no effect in a transgenic mouse model of familial amyotrophic lateral sclerosis. Exp. Neurol. 165, 184–190 (2000).
Guegan, C. et al. Instrumental activation of Bid by caspase-1 in a transgenic mouse model of ALS. Mol. Cell. Neurosci. 20, 553 (2002).
Friedlander, R. M., Brown, R. H., Gagliardini, V., Wang, J. & Yuan, J. Inhibition of ICE slows ALS in mice. Nature 388, 31 (1997).
Zhu, S. et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417, 74–78 (2002). Demonstration that minocycline prevents mitochondrial release of cytochrome c , resulting in a delayed disease onset and extended survival of transgenic ALS mice.
Ishigaki, S. et al. X-Linked inhibitor of apoptosis protein is involved in mutant SOD1- mediated neuronal degeneration. J. Neurochem. 82, 576–584 (2002).
Raoul, C., Henderson, C. E. & Pettmann, B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J. Cell Biol. 147, 1049–1062 (1999).
Raoul, C. et al. Motoneuron death triggered by a specific pathway downstream of Fas. Potentiation by ALS-linked SOD1 mutations. Neuron 35, 1067–1083 (2002).
Nguyen, M. D., Julien, J. P. & Rivest, S. Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: no requirement for proapoptotic interleukin-1β in neurodegeneration. Ann. Neurol. 50, 630–639 (2001).
Brandt, J. et al. Trinucleotide repeat length and clinical progression in Huntington's disease. Neurology 46, 527–531 (1996).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
Thomas, L. B. et al. DNA end labeling (TUNEL) in Huntington's disease and other neuropathological conditions. Exp. Neurol. 133, 265–272 (1995).
Portera-Cailliau, C., Hedreen, J. C., Price, D. L. & Koliatsos, V. E. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J. Neurosci. 15, 3775–3787 (1995).
Ona, V. O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Sanchez, I. et al. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22, 623–633 (1999).
Andreassen, O. A. et al. Malonate and 3-nitropropionic acid neurotoxicity are reduced in transgenic mice expressing a caspase-1 dominant-negative mutant. J. Neurochem. 75, 847–852 (2000).
Duan, W. et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl Acad. Sci. USA 100, 2911–2916 (2003).
Miyashita, T. et al. Expression of extended polyglutamine sequentially activates initiator and effector caspases. Biochem. Biophys. Res. Commun. 257, 724–730 (1999).
Rigamonti, D. et al. Huntingtin's neuroprotective activity occurs via inhibition of procaspase-9 processing. J. Biol. Chem. 276, 14545–14548 (2001).
Goldberg, Y. P. et al. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nature Genet. 13, 442–449 (1996).
Wellington, C. L. et al. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 273, 9158–9167 (1998).
Wellington, C. L. et al. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J. Biol. Chem. 275, 19831–19838 (2000).
Martindale, D. et al. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nature Genet. 18, 150–154 (1998).
DiFiglia, M. et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14, 1075–1081 (1995).
Kim, Y. J. et al. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc. Natl Acad. Sci. USA 98, 12784–12789 (2001).
Mende-Mueller, L. M., Toneff, T., Hwang, S. R., Chesselet, M. F. & Hook, V. Y. Tissue-specific proteolysis of Huntingtin (htt) in human brain: evidence of enhanced levels of N- and C-terminal htt fragments in Huntington's disease striatum. J. Neurosci. 21, 1830–1837 (2001).
Wellington, C. L. et al. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington's disease. J. Neurosci. 22, 7862–7872 (2002).
Gervais, F. G. et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nature Cell Biol. 4, 95–105 (2002).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Loo, D. T. et al. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. Natl Acad. Sci. USA 90, 7951–7955 (1993).
Su, J. H., Anderson, A. J., Cummings, B. J. & Cotman, C. W. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport 5, 2529–2533 (1994).
Stadelmann, C. et al. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am. J. Pathol. 155, 1459–1466 (1999).
Selznick, L. A. et al. In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 58, 1020–1026 (1999).
Rohn, T. T., Head, E., Nesse, W. H., Cotman, C. W. & Cribbs, D. H. Activation of caspase-8 in the Alzheimer's disease brain. Neurobiol. Dis. 8, 1006–1016 (2001).
Rohn, T. T. et al. Caspase-9 Activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol. Dis. 11, 341–354 (2002).
Ivins, K. J., Thornton, P. L., Rohn, T. T. & Cotman, C. W. Neuronal apoptosis induced by β-amyloid is mediated by caspase-8. Neurobiol. Dis. 6, 440–449 (1999).
Giovanni, A. et al. E2F1 mediates death of β-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J. Biol. Chem. 275, 11553–11560 (2000).
Troy, C. M. et al. Caspase-2 mediates neuronal cell death induced by β-amyloid. J. Neurosci. 20, 1386–1392 (2000).
Gervais, F. G. et al. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-β precursor protein and amyloidogenic Aβ peptide formation. Cell 97, 395–406 (1999).
Lu, D. C. et al. A second cytotoxic proteolytic peptide derived from amyloid-β protein precursor. Nature Med. 6, 397–404 (2000).
Langston, J. W., Ballard, P. & Irwin, I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Nicklas, W. J., Yougster, S. K., Kindt, M. V. & Heikkila, R. E. MPTP, MPP+ and mitochondrial function. Life Sci. 40, 721–729 (1987).
Gluck, M. R., Youngster, S. K., Ramsay, R. R., Singer, T. P. & Nicklas, W. J. Studies on the characterization of the inhibitory mechanism of 4'-alkylated 1-methyl-4-phenylpyridinium and phenylpyridine analogues in mitochondria and electron transport particles. J. Neurochem. 63, 655–661 (1994).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Deng, H. -X. et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 261, 1047–1051 (1993).
Chiu, A. Y. et al. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 6, 349–362 (1995).
Dal Canto, M. C. & Gurney, M. E. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res. 676, 25–40 (1995).
Almer, G., Vukosavic, S., Romero, N. & Przedborski, S. Inducible nitric oxide synthase upregulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 72, 2415–2425 (1999).
Tu, P. H. et al. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc. Natl Acad. Sci. USA 93, 3155–3160 (1996).
Teismann, P et al. Cyclooxygenase-2 is instrumental in Parkinson disease neurodegeneration. Proc. Natl Acad. Sci. USA (in the press).
Acknowledgements
We wish to acknowledge the support from the NIH/NINDS, the US Department of Defense, the Lowenstein Foundation, the Lillian Goldman Charitable Trust, the Parkinson's Disease Foundation, the American Parkinson's Disease Association, the Muscular Dystrophy Association, the ALS Association and Project-ALS. We also thank J.P. Vonsattel for his comments.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
OMIM
Glossary
- TUNEL TECHNIQUE
-
This technique enables the visualization of cells undergoing apoptosis by labelling the broken ends of the double-stranded DNA with biotin-conjugated dUTP, using the enzyme terminal deoxynucleotidyl transferase.
- ZYMOGENS
-
The inactive precursors of enzymes — often transformed into the active enzyme by partial proteolysis.
- DOMINANT NEGATIVE
-
A mutant molecule that can form a heteromeric complex with the normal molecule, knocking out the activity of the entire complex.
- GAIN-OF-FUNCTION
-
A mutation that confers either a previously inexistent activity to the affected protein or increases a pre-existing funtion.
- PARAPTOSIS
-
A form of programmed cell death that is related to apoptosis. It is transcription dependent and features swelling of the endoplasmic reticulum and mitochondria. However, it does not depend on caspase activation, except for caspase 9, lacks internucleosomal DNA cleavage, and does not show other morphological hallmarks of apoptosis such as nuclear fragmentation.
Rights and permissions
About this article
Cite this article
Vila, M., Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 4, 365–375 (2003). https://doi.org/10.1038/nrn1100
Issue Date:
DOI: https://doi.org/10.1038/nrn1100
This article is cited by
-
Cellular and Molecular Responses to Mitochondrial DNA Deletions in Kearns-Sayre Syndrome: Some Underlying Mechanisms
Molecular Neurobiology (2024)
-
Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease
npj Parkinson's Disease (2023)
-
Identification of let-7f and miR-338 as plasma-based biomarkers for sporadic amyotrophic lateral sclerosis using meta-analysis and empirical validation
Scientific Reports (2022)
-
Cinnamaldehyde Regulates Insulin and Caspase-3 Signaling Pathways in the Sporadic Alzheimer’s Disease Model: Involvement of Hippocampal Function via IRS-1, Akt, and GSK-3β Phosphorylation
Journal of Molecular Neuroscience (2022)
-
Shared genetic etiology between Parkinson’s disease and blood levels of specific lipids
npj Parkinson's Disease (2021)