Key Points
-
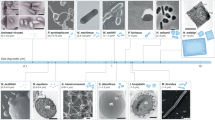
The cell envelope of archaea is fundamentally different from bacteria in that it does not contain peptidoglycan, and archaeal membranes are composed of ether lipids instead of ester lipids.
-
Most archaea are surrounded by a surface-layer (S-layer), which is a proteinaceous two-dimensional crystal layer. Some archaeal cell envelopes contain pseudomurein or other unique sugar polymers.
-
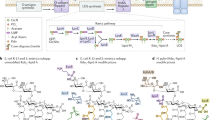
Most of the extracellular archaeal proteins are glycosylated (N-linked, O-linked or both). The archaeal N-glycosylation pathway bears similar features to both the eukaryotic and the bacterial pathway. The known archaeal N-glycans are exceedingly diverse in their composition and structure.
-
Most archaeal pili and all archaeal flagella studied to date are assembled by simple type IV pilin-like machineries.
Abstract
At first glance, archaea and bacteria look alike; however, the composition of the archaeal cell envelope is fundamentally different from the bacterial cell envelope. With just one exception, all archaea characterized to date have only a single membrane and most are covered by a paracrystalline protein layer. This Review discusses our current knowledge of the composition of the archaeal cell surface. We describe the wide range of cell wall polymers, O- and N-glycosylated extracellular proteins and other cell surface structures that archaea use to interact with their environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Woese, C. R. & Fox, G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl Acad. Sci. USA 74, 5088–5090 (1977).
Woese, C. R., Kandler, O. & Wheelis, M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria and Eucarya. Proc. Natl Acad. Sci. USA 87, 4576–4579 (1990).
Houwink, A. L. & Le Poole, J.B. Eine Struktur in der Zellmembran einer Bakterie. Physikalische Verhandlungen 3, 98 (1952).
Kandler, O. & Konig, H. Chemical composition of peptidoglycan free cell walls of methanogenic bacteria. Arch. Microbiol. 118, 141–152 (1978).
Cavicchioli, R. Archaea — timeline of the third domain. Nature Rev. Microbiol. 9, 51–61 (2011).
Beveridge, T. J. Bacterial surface structure, physicochemistry and geo-reactivity. Geochim. Cosmochim. Acta 69, A668 (2005).
Sara, M. & Sleytr, U. B. Crystalline bacterial cell surface layers (S-layers): from cell structure to biomimetics. Prog. Biophys. Mol. Biol. 65, 83–111 (1996). A comprehensive overview of bacterial crystalline S-layer proteins, also giving insights into their properties for nanobiotechnological applications
Houwink, A. L. Flagella, gas vacuoles and cell-wall structure in Halobacterium halobium; an electron microscope study. J. Gen. Microbiol. 15, 146–150 (1956). Historical electron microscope study describing the first two-dimensional hexagonal crystal lattice of an S-layer
Grogan, D. W. Isolation and fractionation of cell envelope from the extreme thermoacidophile Sulfolobus acidocaldarius. J. Microbiol. Methods 26, 35–43 (1996).
Veith, A. et al. Acidianus, Sulfolobus and Metallosphaera surface layers: structure, composition and gene expression. Mol. Microbiol. 73, 58–72 (2009).
Beveridge, T. J., Patel, G. B., Harris, B. J. & Sprott, G. D. The ultrastructure of Methanothrix concilii, a mesophilic aceticlastic methanogen. Can. J. Microbiol. 32, 703–710 (1986).
Zeikus, J. G. & Bowen, V. G. Fine structure of Methanospirillum hungatii. J. Bacteriol. 121, 373–380 (1975).
Beveridge, T. J. & Graham, L. L. Surface layers of bacteria. Microbiol. Mol. Biol. Rev. 55, 684–705 (1991).
Beveridge, T. Jv., Stewart, M., Doyle, R. J. & Sprott, G. D. Unusual stability of the Methanospirillum hungatei sheath. J. Bacteriol. 162, 728–737 (1985).
Firtel, M., Southam, G., Harauz, G. & Beveridge, T. J. Characterization of the cell wall of the sheathed methanogen Methanospirillum hungatei Gp1 as an S-layer. J. Bacteriol. 175, 7550–7560 (1993).
Sprott, G. D., Colvin, J. R. & Mckellar, R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can. J. Microbiol. 25, 730–738 (1979).
Zehnder, A. J. B., Huser, B. A., Brock, T. D. & Wuhrmann, K. Characterization of an acetate decarboxylating, non hydrogen oxidizing methane bacterium. Arch. Microbiol. 124, 1–11 (1980).
Shaw, P. J., Hills, G. J., Henwood, J. A., Harris, J. E. & Archer, D. B. Three-dimensional architecture of the cell sheath and septa of Methanospirillum hungatei. J. Bacteriol. 161, 750–757 (1985).
Beveridge, T. J. Use of the Gram stain in microbiology. Biotech. Histochem. 76, 111–118 (2001).
Messner, P. & Sleytr, U. B. Asparaginyl-rhamnose:a novel type of protein-carbohydrate linkage in a eubacterial surface-layer glycoprotein. FEBS Lett. 228, 317–320 (1988).
Messner, P., Pum, D. & Sleytr, U. B. Characterization of the ultrastructure and the self-Assembly of the surface-layer of Bacillus stearothermophilus strain Nrs 2004/3a. J. Ultrastruct. Mol. Struct. Res. 97, 73–88 (1986).
Kandler, O. & Koenig, H. in The Biochemistry of Archaea (Archaebacteria) (eds M. Kates et al.) 223-333 (Elsevier, the Netherlands, 1993). An excellent and insightful overview of the different cell envelopes among the Archaea.
Konig, H., Hartmann, E. & Karcher, U. Pathways and principles of the biosynthesis of methanobacterial cell wall polymers. Syst. Appl. Microbiol. 16, 510–517 (1994).
Scheffers, D. J. & Pinho, M. G. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69, 585–607 (2005).
Claus, H. & Koenig, H. (eds) 231-251 Cell Envelopes of Methanogens (Springer, Berlin, 2010).
Kreisl, P. & Kandler, O. Chemical structure of the cell wall polymer of Methanosarcina. Syst. Appl. Microbiol. 7, 293–299 (1986).
Sowers, K. R., Boone, J. E. & Gunsalus, R. P. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59, 3832–3839 (1993).
Kjellen, L. & Lindahl, U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60, 443–475 (1991).
Hartmann, E. & Konig, H. Nucleotide-activated oligosaccharides are intermediates of the cell wall polysaccharide of Methanosarcina barkeri. Biol. Chem. Hoppe Seyler 372, 971–974 (1991).
Tindall, B. J., Ross, H. N. M. & Grant, W. D. Natronobacterium gen. nov. and Natronococcus gen. nov., 2 new genera of haloalkaliphilic archaebacteria. Syst. Appl. Microbiol. 5, 41–57 (1984).
Niemetz, R., Karcher, U., Kandler, O., Tindall, B. J. & Konig, H. The cell wall polymer of the extremely halophilic archaeon Natronococcus occultus. Eur. J. Biochem. 249, 905–911 (1997).
Kocur, M., Martinec, T. & Smid, B. Fine structure of extreme halophilic cocci. Microbios 5, 101–107 (1972).
Steber, J. & Schleifer, K. H. N-glycylglucosamine: a novel constituent in the cell wall of Halococcus morrhuae. Arch. Microbiol. 123, 209–212 (1979).
Schleifer, K. H., Steber, J. & Mayer, H. Chemical composition and structure of the cell wall of Halococcus morrhuae. Zentralblatt. Bakteriol. Parasitenkd Infekt. Hyg. C3, 171–178 (1982).
Steber, J. & Schleifer, K. H. Halococcus morrhuae: a sulfated heteropolysaccharide as structural component of bacterial cell wall. Arch. Microbiol. 105, 173–177 (1975).
Bolhuis, H. et al. The genome of the square archaeon Haloquadratum walsbyi: life at the limits of water activity. BMC Genomics 7, 169 (2006).
Ashiuchi, M. & Misono, H. Biochemistry and molecular genetics of poly-γ-glutamate synthesis. Appl. Microbiol. Biotechnol. 59, 9–14 (2002).
Hollingsworth, M. A. & Swanson, B. J. Mucins in cancer: Protection and control of the cell surface. Nature Rev. Cancer 4, 45–60 (2004).
Golyshina, O. V. & Timmis, K. N. Ferroplasma and relatives, recently discovered cell wall-lacking archaea making a living in extremely acid, heavy metal-rich environments. Environ. Microbiol. 7, 1277–1288 (2005).
Darland, G., Brock, T. D., Samsonoff, W. & Conti, S. F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science 170, 1416–1418 (1970).
Segerer, A., Langworthy, T. A. & Stetter, K. O. Thermoplasma acidophilum and Thermoplasma volcanium spp. nov. from solfatara fields. Syst. Appl. Microbiol. 10, 161–171 (1988).
Yang, L. L. & Haug, A. Purification and partial characterization of a procaryotic glycoprotein from the plasma membrane of Thermoplasma acidophilum. Biochim. Biophys. Acta 556, 265–277 (1979).
Smith, P. F. Lipoglycans from Mycoplasmas. Crit. Rev. Microbiol. 11, 157–186 (1984).
Langworthy, T. A. Lipids of archaebacteria — extreme halophiles, methanogens and thermoacidophiles. J. Am. Oil. Chem. Soc. 59, A285 (1982).
Rachel, R., Wyschkony, I., Riehl, S. & Huber, H. The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea 1, 9–18 (2002).
Burghardt, T., Nather, D. J., Junglas, B., Huber, H. & Rachel, R. The dominating outer membrane protein of the hyperthermophilic archaeum Ignicoccus hospitalis: a novel pore-forming complex. Mol. Microbiol. 63, 166–176 (2007).
Kuper, U., Meyer, C., Muller, V., Rachel, R. & Huber, H. Energized outer membrane and spatial separation of metabolic processes in the hyperthermophilic Archaeon Ignicoccus hospitalis. Proc. Natl Acad. Sci. USA 107, 3152–3156 (2010).
Soler, N., Marguet, E., Verbavatz, J. M. & Forterre, P. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. Microbiol. 159, 390–399 (2008).
Ellen, A. F. et al. Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles 13, 67–79 (2009).
Reysenbach, A. L. et al. A ubiquitous thermoacidophilic archaeon from deep-sea hydrothermal vents. Nature 442, 444–447 (2006).
Zeitler, R., Hochmuth, E., Deutzmann, R. & Sumper, M. Exchange of Ser4 for Val, Leu or Asn in the sequon AsnAlaSer does not prevent N-glycosylation of the cell surface glycoprotein from Halobacterium halobium. Glycobiology 8, 1157–1164 (1998).
Voisin, S. et al. Identification and characterization of the unique N-linked glycan common to the flagellins and S-layer glycoprotein of Methanococcus voltae. J. Biol. Chem. 280, 16586–16593 (2005).
Sumper, M., Berg, E., Mengele, R. & Strobel, I. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J. Bacteriol. 172, 7111–7118 (1990).
Paul, G., Lottspeich, F. & Wieland, F. Asparaginyl-N-Acetylgalactosamine. Linkage unit of halobacterial glycosaminoglycan. J. Biol. Chem. 261, 1020–1024 (1986).
Mescher, M. F. & Strominger, J. L. Purification and characterization of a prokaryotic glycoprotein from the cell-envelope of Halobacterium salinarium. J. Biol. Chem. 251, 2005–2014 (1976). The first report of a glycosylated prokaryotic protein.
Kessel, M., Volker, S., Santarius, U., Huber, R. & Baumeister, W. 3-Dimensional reconstruction of the surface protein of the extremely thermophilic archaebacterium Archaeoglobus fulgidus. Syst. Appl. Microbiol. 13, 207–213 (1990).
Kessel, M., Wildhaber, I., Cohen, S. & Baumeister, W. 3-Dimensional structure of the regular surface glycoprotein layer of Halobacterium volcanii from the Dead-Sea. EMBO J. 7, 1549–1554 (1988).
Peyfoon, E. et al. The S-layer glycoprotein of the crenarchaeote Sulfolobus acidocaldarius is glycosylated at multiple sites with chitobiose-linked N-glycans. Archaea 29 Sep 2010 (doi:10.1155/2010/754101).
Ng, S., Chaban, B. & Jarrell, K. Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications. J. Mol. Microbiol. Biotechnol. 11, 167–191 (2006).
Ng, S. Y. et al. Genetic and mass spectrometry analysis of the unusual type IV-like pili of the archaeon Methanococcus maripaludis. J. Bacteriol. 193, 804–814 (2011).
Elferink, M. G., Albers, S. V., Konings, W. N. & Driessen, A. J. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol. 39, 1494–1503 (2001).
Kikuchi, A., Sagami, H. & Ogura, K. Evidence for covalent attachment of diphytanylglyceryl phosphate to the cell-surface glycoprotein of Halobacterium halobium. J. Biol. Chem. 274, 18011–18016 (1999).
Konrad, Z. & Eichler, J. Lipid modification of proteins in Archaea: attachment of a mevalonic acid-based lipid moiety to the surface-layer glycoprotein of Haloferax volcanii follows protein translocation. Biochem. J. 366, 959–964 (2002).
Chaban, B., Logan, S. M., Kelly, J. F. & Jarrell, K. F. AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae. J. Bacteriol. 191, 187–195 (2009).
Kelly, J., Logan, S. M., Jarrell, K. F., VanDyke, D. J. & Vinogradov, E. A novel N-linked flagellar glycan from Methanococcus maripaludis. Carbohydr. Res. 344, 648–653 (2009).
VanDyke, D. J. et al. Identification of a putative acetyltransferase gene, MMP0350, which affects proper assembly of both flagella and pili in the archaeon Methanococcus maripaludis. J. Bacteriol. 190, 5300–5307 (2008).
Yurist-Doutsch, S. et al. N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol. Microbiol. 75, 1047–1058 (2010).
Magidovich, H. et al. AglP is a S-adenosyl-L-methionine-dependent-methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol. Microbiol. 76, 190–199 (2010).
Abu-Qarn, M. et al. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 374, 1224–1236 (2007).
Nita-Lazar, M., Wacker, M., Schegg, B., Amber, S. & Aebi, M. The N-X-S/T consensus sequence is required but not sufficient for bacterial N-linked protein glycosylation. Glycobiology 15, 361–367 (2005).
Glover, K. J., Weerapana, E., Numao, S. & Imperiali, B. Chemoenzymatic synthesis of glycopeptides with PglB, a bacterial oligosaccharyl transferase from Campylobacter jejuni. Chem. Biol. 12, 1311–1315 (2005).
Schwarz, F. et al. A combined method for producing homogeneous glycoproteins with eukaryotic N-glycosylation. Nature Chem. Biol. 6, 264–266 (2010).
Zufferey, R. et al. Stt3, a highly conserved protein required for yeast oligosaccharyl transferase activity in vivo. EMBO J. 14, 4949–4960 (1995).
Yan, Q., Prestwich, G. D. & Lennarz, W. J. The Ost1p subunit of yeast oligosaccharyl transferase recognizes the peptide glycosylation site sequence, AsnX-Ser/Thr. J. Biol. Chem. 274, 5021–5025 (1999).
Dempski, R. E. & Imperiali, B. Heterologous expression and biophysical characterization of soluble oligosaccharyl transferase subunits. Arch. Biochem. Biophys. 431, 63–70 (2004).
Igura, M. et al. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 27, 234–243 (2008).
Brockl, G. et al. Analysis and nucleotide sequence of the genes encoding the surface layer glycoproteins of the hyperthermophilic methanogens Methanothermus fervidus and Methanothermus sociabilis. Eur. J. Biochem. 199, 147–152 (1991).
Karcher, U. et al. Primary structure of the heterosaccharide of the surface glycoprotein of Methanothermus fervidus. J. Biol. Chem. 268, 26821–26826 (1993).
Nusser, E. & Konig, H. S-layer studies on 3 species of Methanococcus living at different temperatures. Can. J. Microbiol. 33, 256–261 (1987).
Engelhardt, H. & Peters, J. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J. Struct. Biol. 124, 276–302 (1998).
Mengele, R. & Sumper, M. Drastic differences in glycosylation of related S-layer glycoproteins from moderate and extreme halophiles. J. Biol. Chem. 267, 8182–8185 (1992).
Paul, G. & Wieland, F. Sequence of the halobacterial glycosaminoglycan. J. Biol. Chem. 262, 9587–9593 (1987).
Magidovich, H. & Eichler, J. Glycosyltransferases and oligosaccharyltransferases in Archaea: putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol. Lett. 300, 122–130 (2009).
Maita, N., Nyirenda, J., Igura, M., Kamishikiryo, J. & Kohda, D. Comparative structural biology of eubacterial and archaeal oligosaccharyltransferases. J. Biol. Chem. 285, 4941–4950 (2010).
Nothaft, H. & Szymanski, C. M. Protein glycosylation in bacteria: sweeter than ever. Nature Rev. Microbiol. 8, 765–778 (2010).
Stimson, E. et al. Meningococcal pilin: a glycoprotein substituted with digalactosyl-2, 4-diacetamido-2, 4, 6-trideoxyhexose. Mol. Microbiol. 17, 1201–1214 (1995).
Thibault, P. et al. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276, 34862–34870 (2001).
Schirm, M. et al. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 48, 1579–1592 (2003).
Grubman, A. et al. Vitamin B6 is required for full motility and virulence in Helicobacter pylori. MBio 1, e00112–10 (2010).
Virji, M. et al. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5, 1831–1841 (1991).
Hettmann, T. et al. Cytochrome b558/566 from the archaeon Sulfolobus acidocaldarius. J. Biol. Chem. 273, 12032–12040 (1998).
Koning, S. M., Albers, S. V., Konings, W. N. & Driessen, A. J. Sugar transport in (hyper)thermophilic archaea. Res. Microbiol. 153, 61–67 (2002).
Albers, S. V., Koning, S. M., Konings, W. N. & Driessen, A. J. Insights into ABC transport in archaea. J. Bioenerg. Biomembr. 36, 5–15 (2004).
Antón, J., Meseguer, I. & Rodríguez-Valera, F. Production of an extracellular polysaccharide by Haloferax mediterranei. Appl. Environ. Microbiol. 54, 2381–2386 (1988).
Rinker, K. D. & Kelly, R. M. Growth physiology of the hyperthermophilic Archaeon Thermococcus litoralis: development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl. Environ. Microbiol. 62, 4478–4485 (1996).
Paramonov, N. A. et al. The structure of the exocellular polysaccharide produced by the Archaeon Haloferax gibbonsii (ATCC 33959). Carbohydr. Res. 309, 89–94 (1998).
Parolis, L. A. et al. Structural studies on the acidic exopolysaccharide from Haloferax denitrificans ATCC 35960. Carbohydr. Res. 319, 133–140 (1999).
Nicolaus, B., Manca, M. C., Romano, I. & Lama, L. Production of an exopolysaccharide from two thermophilic archaea belonging to the genus Sulfolobus. FEMS Microbiol. Lett. 109, 203–206 (2003).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nature Rev. Microbiol. 8, 623–633 (2010).
Zolghadr, B. et al. Appendage mediated surface adherence of Sulfolobus solfataricus. J. Bacteriol. 192, 104–110 (2010).
Koerdt, A., Godeke, J., Berger, J., Thormann, K. M. & Albers, S. V. Crenarchaeal biofilm formation under extreme conditions. PLoS ONE 5, e14104 (2010).
Stetter, K. O., Konig, H. & Stackebrandt, E. Pyrodictium gen. nov., a new genus of submarine disc shaped sulfur reducing archaebacteria growing optimally at 105 °C. Syst. Appl. Microbiol. 4, 535–551 (1983).
Horn, C., Paulmann, B., Kerlen, G., Junker, N. & Huber, H. In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope. J. Bacteriol. 181, 5114–5118 (1999).
Rieger, G. et al. Ultrastructure of Pyrodictium cells and extracellular tubules, analysed by TEM and SEM. Eur. J. Cell Biol. 74, 96–96 (1997).
Nickell, S., Hegerl, R., Baumeister, W. & Rachel, R. Pyrodictium cannulae enter the periplasmic space but do not enter the cytoplasm, as revealed by cryo-electron tomography. J. Struct. Biol. 141, 34–42 (2003).
Moissl, C., Rachel, R., Briegel, A., Engelhardt, H. & Huber, R. The unique structure of archaeal 'hami', highly complex cell appendages with nano-grappling hooks. Mol. Microbiol. 56, 361–370 (2005).
Rudolph, C., Wanner, G. & Huber, R. Natural communities of novel archaea and bacteria growing in cold sulfurous springs with a string-of-pearls-like morphology. Appl. Environ. Microbiol. 67, 2336–2344 (2001).
Rieger, G., Rachel, R., Hermann, R. & Stetter, K. O. Ultrastructure of the hyperthermophilic Archaeon Pyrodictium abyssi. J. Struct. Biol. 115, 78–87 (1995).
Thoma, C. et al. The Mth60 fimbriae of Methanothermobacter thermoautotrophicus are functional adhesins. Environ. Microbiol. 10, 2785–2795 (2008).
Kalmokoff, M. L. & Jarrell, K. F. Cloning and sequencing of a multigene family encoding the flagellins of Methanococcus voltae. J. Bacteriol. 173, 7113–7125 (1991). First report showing that archaeal flagellins have class III signal peptides and are therefore structurally linked to type IV pili.
Szabo, Z. et al. Identification of diverse archaeal proteins with class III signal peptides cleaved by distinct archaeal prepilin peptidases. J. Bacteriol. 189, 772–778 (2007). Bioinformatics were used to identify a multitude of possible type IV pilins in archaeal genomes.
Strom, M. S., Nunn, D. N. & Lory, S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl Acad. Sci. USA 90, 2404–2408 (1993).
Albers, S. V., Szabo, Z. & Driessen, A. J. Archaeal homolog of bacterial type IV prepilin signal peptidases with broad substrate specificity. J. Bacteriol. 185, 3918–3925 (2003).
Bardy, S. L. & Jarrell, K. F. FlaK of the archaeon Methanococcus maripaludis possesses preflagellin peptidase activity. FEMS Microbiol. Lett. 208, 53–59 (2002).
Bardy, S. L. & Jarrell, K. F. Cleavage of preflagellins by an aspartic acid signal peptidase is essential for flagellation in the archaeon Methanococcus voltae. Mol. Microbiol. 50, 1339–1347 (2003).
Szabo, Z., Albers, S. V. & Driessen, A. J. Active-site residues in the type IV prepilin peptidase homologue PibD from the archaeon Sulfolobus solfataricus J. Bacteriol. 188, 1437–1443 (2006).
Muller, D. W. et al. The Iho670 fibers of Ignicoccus hospitalis: a new type of archaeal cell surface appendage. J. Bacteriol. 191, 6465–6468 (2009).
Frols, S. et al. Response of the hyperthermophilic archaeon Sulfolobus solfataricus to UV damage. J. Bacteriol. 189, 8708–8718 (2007).
Gotz, D. et al. Responses of hyperthermophilic crenarchaea to UV irradiation. Genome Biol. 8, R220 (2007).
Frols, S. et al. UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation. Mol. Microbiol. 70, 938–952 (2008).
Albers, S. V. et al. Glucose transport in the extremely thermoacidophilic Sulfolobus solfataricus involves a high-affinity membrane-integrated binding protein. J. Bacteriol. 181, 4285–4291 (1999).
Zolghadr, B., Weber, S., Szabo, Z., Driessen, A. J. & Albers, S. V. Identification of a system required for the functional surface localization of sugar binding proteins with class III signal peptides in Sulfolobus solfataricus. Mol. Microbiol. 64, 795–806 (2007).
Zolghadr, B., Klingl, A., Rachel, R., Driessen, A. J. & Albers, S. V. The bindosome is a structural component of the Sulfolobus solfataricus cell envelope. Extremophiles 15, 235–244 (2011).
Ng, S. Y., Chaban, B. & Jarrell, K. F. Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications. J. Mol. Microbiol. Biotechnol. 11, 167–191 (2006).
Ng, S. Y., Zolghadr, B., Driessen, A. J., Albers, S. V. & Jarrell, K. F. Cell surface structures of archaea. J. Bacteriol. 190, 6039–6047 (2008).
Marwan, W., Alam, M. & Oesterhelt, D. Rotation and switching of the flagellar motor assembly in Halobacterium halobium. J. Bacteriol. 173, 1971–1977 (1991).
Streif, S., Staudinger, W. F., Marwan, W. & Oesterhelt, D. Flagellar rotation in the archaeon Halobacterium salinarum depends on ATP. J. Mol. Biol. 384, 1–8 (2008). This study demonstrated that archaeal flagella movement is driven by ATP hydrolysis and not by the proton motive force.
Schlesner, M. et al. Identification of Archaea-specific chemotaxis proteins which interact with the flagellar apparatus. BMC Microbiol. 9, 56 (2009).
Chaban, B. et al. Systematic deletion analyses of the fla genes in the flagella operon identify several genes essential for proper assembly and function of flagella in the archaeon Methanococcus maripaludis. Mol. Microbiol. 66, 596–609 (2007).
Henneberger, R., Moissl, C., Amann, T., Rudolph, C. & Huber, R. New insights into the lifestyle of the cold-loving SM1 euryarchaeon: natural growth as a monospecies biofilm in the subsurface. Appl. Environ. Microbiol. 72, 192–199 (2006).
Nather, D. J., Rachel, R., Wanner, G. & Wirth, R. Flagella of Pyrococcus furiosus: multifunctional organelles, made for swimming, adhesion to various surfaces, and cell-cell contacts. J. Bacteriol. 188, 6915–6923 (2006).
Tripepi, M., Imam, S. & Pohlschroder, M. Haloferax volcanii flagella are required for motility but are not involved in PibD-dependent surface adhesion. J. Bacteriol. 192, 3093–3102 (2010).
Bettstetter, M., Peng, X., Garrett, R. A. & Prangishvili, D. AFV1, a novel virus infecting hyperthermophilic archaea of the genus Acidianus. Virology 315, 68–79 (2003).
Pyatibratov, M. G. et al. Alternative flagellar filament types in the haloarchaeon Haloarcula marismortui. Can. J. Microbiol. 54, 835–844 (2008).
Leigh, J. A., Albers, S. V., Atomi, H. & Allers, T. Model organisms for genetics in the domain archaea: methanogens, halophiles, Thermococcales and Sulfolobales. FEMS Microbiol. Rev. 7 Mar 2011 (doi: 10.1111/j. 1574-69762011.00265.x).
Samson, R. Y., Obita, T., Freund, S. M., Williams, R. L. & Bell, S. D. A role for the ESCRT system in cell division in archaea. Science 322, 1710–1713 (2008).
Lindas, A. C., Karlsson, E. A., Lindgren, M. T., Ettema, T. J. & Bernander, R. A unique cell division machinery in the Archaea. Proc. Natl Acad. Sci. USA 105, 18942–18946 (2008). References 137 and 138 demonstrate that the ESCRTIII proteins localize to the mid-cell during crenarchaeal cell division.
Makarova, K. S., Yutin, N., Bell, S. D. & Koonin, E. V. Evolution of diverse cell division and vesicle formation systems in Archaea. Nature Rev. Microbiol. 8, 731–741 (2010).
Wirth, R. et al. The mode of cell wall growth in selected Archaea follows the general mode of cell wall growth in Bacteria — an analysis using fluorescent dyes. Appl. Environ. Microbiol. 77, 1556–1562 (2010).
Kates, M. Archaebacterial lipids — structure, biosynthesis and function. Biochem. Soc. Symp. 51–72 (1992).
Brochier-Armanet, C., Boussau, B., Gribaldo, S. & Forterre, P. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nature Rev. Microbiol. 6, 245–252 (2008).
Elkins, J. G. et al. A korarchaeal genome reveals insights into the evolution of the Archaea. Proc. Natl Acad. Sci. USA 105, 8102–8107 (2008).
Rachel, R. (ed.) Ch. 9 Cell Envelopes of Crenarchaeota and Nanoarchaeota (Springer, Berlin, 2010).
Baumeister, W., Wildhaber, I. & Phipps, B. M. Principles of organization in eubacterial and archaebacterial surface-proteins. Can. J. Microbiol. 35, 215–227 (1989).
Peters, J. et al. Tetrabrachion: a filamentous archaebacterial surface protein assembly of unusual structure and extreme stability. J. Mol. Biol. 245, 385–401 (1995).
Pruschenk, R. & Baumeister, W. 3-Dimensional structure of the surface protein of Sulfolobus solfataricus. Eur. J. Cell Biol. 45, 185–191 (1988).
Wildhaber, I., Santarius, U. & Baumeister, W. 3-Dimensional structure of the surface protein of Desulfurococcus mobilis. J. Bacteriol. 169, 5563–5568 (1987).
Messner, P., Pum, D., Sara, M., Stetter, K. O. & Sleytr, U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J. Bacteriol. 166, 1046–1054 (1986).
Wildhaber, I. & Baumeister, W. The cell envelope of Thermoproteus tenax: 3-Dimensional structure of the surface-layer and its role in shape maintenance. EMBO J. 6, 1475–1480 (1987).
Haeuptle, M. A. & Hennet, T. Congenital disorders of glycosylation: an update on defects affecting the biosynthesis of dolichol-linked oligosaccharides. Hum. Mutat. 30, 1628–1641 (2009).
Weerapana, E. & Imperiali, B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology 16, 91R–101R (2006).
Van Dyke, D. J. et al. Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 72, 633–644 (2009).
Calo, D., Kaminski, L. & Eichler, J. Protein glycosylation in Archaea: sweet and extreme. Glycobiology 20, 1065–1076 (2010). Recent review of N -glycosylation in archaea, summarizing the three glycosylation pathways in archaea that have been studied so far.
Young, N. M. et al. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 277, 42530–42539 (2002).
Szymanski, C. M. & Wren, B. W. Protein glycosylation in bacterial mucosal pathogens. Nature Rev. Microbiol. 3, 225–237 (2005).
Bellack, A., Huber, H., Rachel, R., Wanner, G. & Wirth, R. Methanocaldococcus villosus sp. nov., a heavily flagellated archaeon adhering to surfaces and forming cell-cell contacts. Int. J. Syst. Evol. Microbiol. 9 Jul 2010 (doi:10.1099/ijs.0.023663-0).
Acknowledgements
B.H.M. and S.-V.A. were supported by a VIDI grant of the Dutch Science Organization (NWO) and S.-V.A. received additional intramural funds from the Max Planck Society. We want to thank R. Rachel, C. Moissl and G. Wanner for providing us with unpublished picture material. We thank A. Bozarth for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1 (table)
Summary of occurrence of S-layer proteins and cell wall polymers in Archaea. (PDF 206 kb)
Supplementary information S2 (table)
Extracellular sugar polymers in Archaea. (PDF 293 kb)
Related links
Glossary
- Black smoker
-
A type of hydrothermal vent, which appears as a black chimney-like structure that emits a cloud of black material composed of high levels of sulphur-bearing minerals, or sulphides.
- Oblique symmetry
-
A form of symmetry displayed by S-layer proteins, in which the proteins do not lie at a right angle, or multiples of a right angle, to each other.
- Square symmetry
-
A form of symmetry displayed by S-layer proteins, in which the proteins lie at a right angle, or multiples of a right angle, to each other.
- Hexagonal symmetry
-
A form of symmetry displayed by S-layer proteins, in which the proteins are at an angle of 60° or 120° to each other.
- Paracrystalline
-
A lattice structure that is highly ordered over short distances but lacks long-range ordering at least in one direction.
- D-amino acids
-
All amino acids, except glycine, can exist as either one of two optical isomers, which are mirror images of each other. These forms are called L- or D-amino acids. Only L-amino acids can be recognized in the translation process to be used for the synthesis of proteins. D-amino acids are more rare and can be found, for example, in bacterial peptidoglycan.
- Alkaliphilic
-
Microorganisms that thrive in alkaline environments and require a pH higher than 9 for growth.
- Halophilic
-
Microorganisms that require high concentrations of salt for growth.
- Glycocalyx
-
A cell-surface coat made of glycoproteins and glycolipids.
- Thermoadaptation
-
A mechanism to enable the growth of organisms at high temperatures including adaptation of, for example, proteins, lipids and other cellular components.
- Hyperthermophilic
-
Microorganisms that require high temperatures above 80 °C for optimal growth.
- Signal peptide
-
Part of a preprotein that targets itself to the secretion machinery in the cytoplasmic membrane.
Rights and permissions
About this article
Cite this article
Albers, SV., Meyer, B. The archaeal cell envelope. Nat Rev Microbiol 9, 414–426 (2011). https://doi.org/10.1038/nrmicro2576
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2576
This article is cited by
-
Cell cycle dependent coordination of surface layer biogenesis in Caulobacter crescentus
Nature Communications (2024)
-
The cell biology of archaea
Nature Microbiology (2022)
-
Cell division in the archaeon Haloferax volcanii relies on two FtsZ proteins with distinct functions in division ring assembly and constriction
Nature Microbiology (2021)
-
The archaeal protein SepF is essential for cell division in Haloferax volcanii
Nature Communications (2021)
-
Comparative genomic analysis of Methanimicrococcus blatticola provides insights into host adaptation in archaea and the evolution of methanogenesis
ISME Communications (2021)