Key Points
-
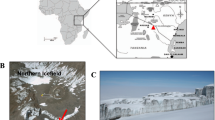
Approximately 0.3% of Antarctica is perpetually ice free, with the largest single area being the McMurdo Dry Valleys in Victoria Land. These glacially carved valleys are thought to be some of the coldest, driest places on earth and analogues of Martian terrestrial environments.
-
The valleys are dominated by glacially derived mineral soils, which are characterized by large annual and daily temperature fluctuations, sever aridity, extreme oligotrophy and, often, high salinity.
-
Eukaryotic and prokaryotic diversity analyses in the Dry Valleys suggests that these soils constitute one of the simplest trophic structures in the terrestrial biosphere.
-
Modern molecular tools have recently revealed that the Dry Valley soils harbour an extraordinary diversity of taxonomically unique bacteria, the communities of which seem to be structured primarily by abiotic forces. In addition, these studies are finding that the environment, which outwardly seems to be mundane and homogeneous, supports highly heterogeneous microbial communities.
-
The extreme nature of the Dry Valley environment has led to the development of microbial communities in protected niches. The most substantial of these are the lithic environments, where well-developed microbial communities have colonized cracks in rocks (these communities are known as chasmoliths), the undersides of translucent rocks (hypoliths) and the subsurface interstices of coarse crystalline rock types (endoliths).
-
Climate warming and cooling have both contributed to substantial changes in the structure and function of ecosystems in the Dry Valleys, in particular of soils that seem to be especially sensitive to climate change and variability. A decadal-scale cooling between 1986 and 2001 was associated with a 60% decline in the dominant invertebrate species, and a summer warming and melting event in January 2002 stimulated soil diversity and microbial biomass.
Abstract
The arid soils of the Antarctic Dry Valleys constitute some of the oldest, coldest, driest and most oligotrophic soils on Earth. Early studies suggested that the Dry Valley soils contained, at best, very low levels of viable microbiota. However, recent applications of molecular methods have revealed a dramatically contrasting picture — a very wide diversity of microbial taxa, many of which are uncultured and taxonomically unique, and a community that seems to be structured solely by abiotic processes. Here we review our understanding of these extreme Antarctic terrestrial microbial communities, with particular emphasis on the factors that are involved in their development, distribution and maintenance in these cold desert environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boyd, W. L., Staley, J. T. & Boyd, J. W. in Antarctic Soils and Soil Forming Processes. Antarctic Research Series 125–159 (American Geophysical Union, Washington DC, 1966).
Bockheim, J. G. Functional diversity of soils along environmental gradients in the Ross Sea region, Antarctica. Geoderma 144, 32–42 (2008).
Vincent, W. F. Microbial Ecosystems of Antarctica (Cambridge Univ. Press, Cambridge, UK, 1988).
Doran, P. T. et al. Antarctic climate cooling and terrestrial ecosystem response. Nature 415, 517–520 (2002).
Aislabie, J. et al. Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Biol. Biochem. 38, 3041–3056 (2006).
Dana, G. L., Wharton, R. A. & Dubayah, R. in Ecosystem Dynamics in A Polar Desert: the Mcmurdo Dry Valleys, Antarctica. Antarctic Research Series 39–64 (American Geophysical Union, Washington DC, 1998).
Poage, M. A., Barrettt, J. E., Virginia, R. A. & Wall, D. H. The influence of soil geochemistry on nematode distribution, McMurdo Dry Valleys, Antarctica. Arct. Antarct. Alp. Res. 40, 119–128 (2008).
Smith, R. C. et al. Ozone depletion — ultraviolet-radiation and phytoplankton biology in Antarctic waters. Science 255, 952–959 (1992).
Tosi, S., Onofri, S., Brusoni, M., Zucconi, L. & Vishniac, H. Response of Antarctic soil fungal assemblages to experimental warming and reduction of UV radiation. Polar Biol. 28, 470–482 (2005).
Vishniac, H. S. in Antarctic Microbiology 297–341 (Wiley-Liss, New York, 1993).
Claridge, G. G. C. & Campbell, I. B. The salts in Antarctic soils, their distribution and relationship to soil processes. Soil Sci. 123, 377–384 (1977).
Bockheim, J. G. Properties and classification of cold desert soils from Antarctica. Soil Sci. Soc. Am. J. 61, 224–231 (1997).
Treonis, A. M., Wall, D. H. & Virginia, R. A. The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Funct. Ecol. 14, 460–467 (2000).
Scott, R. F. The Voyage of the Discovery Vol. 1 (Smith, Elder & Co., London, UK, 1905).
Horowitz, N. H., Cameron, R. E. & Hubbard, J. S. Microbiology of the Dry Valleys of Antarctica. Science 176, 242–245 (1972).
Smith, J. J., Ah Tow, L., Stafford, W., Cary, C. & Cowan, D. A. Bacterial diversity in three different Antarctic cold desert mineral soils. Microb. Ecol. 51, 413–421 (2006). The first application of molecular tools to demonstrate that the microbial diversity in Dry Valley soils is higher than was expected.
Barrett, J. E. et al. Co-variation in soil biodiversity and biogeochemistry in northern and southern Victoria Land, Antarctica. Antarct. Sci. 18, 535–548 (2006).
Yergeau, E., Newsham, K. K., Pearce, D. A. & Kowalchuk, G. A. Patterns of bacterial diversity across a range of Antarctic terrestrial habitats. Environ. Microbiol. 9, 2670–2682 (2007). An excellent paper looking at the diversity of soil bacteria across a broad latitudinal gradient of Antarctic Peninsula environments.
Niederberger, T. D. et al. Microbial community composition in soils of Northern Victoria Land, Antarctica. Environ. Microbiol. 10, 1713–1724 (2008).
Cowan, D., Russell, N., Mamais, A. & Sheppard, D. Antarctic Dry Valley mineral soils contain unexpectedly high levels of microbial biomass. Extremophiles 6, 431–436 (2002).
Barrett, J. E. et al. Persistent effects of a discrete warming event on a polar desert ecosystem. Glob. Chang. Biol. 14, 2249–2261 (2008).
Barrett, J. E., Virginia, R. A., Wall, D. H. & Adams, B. J. Decline in a dominant invertebrate species contributes to altered carbon cycling in a low-diversity soil ecosystem. Glob. Chang. Biol. 14, 1734–1744 (2008).
Witherow, R. A. et al. The aeolian flux of calcium, chloride and nitrate to the McMurdo Dry Valleys landscape: evidence from snow pit analysis. Antarct. Sci. 18, 497–505 (2006).
Chinn, T. H. in Physical and Biogeochemical Processes in Antarctic Lakes 1–51 (American Geophysical Union, Washington DC, 1993).
Nylen, T. H., Fountain, A. G. & Doran, P. T. Climatology of katabatic winds in the McMurdo dry valleys, southern Victoria Land, Antarctica. J. Geophys. Res. 109, D03114 (2004).
Bockheim, J. G. Landform and soil development in the McMurdo Dry Valleys, Antarctica: a regional synthesis. Arct. Antarct. Alp. Res. 34, 308–317 (2002).
Hagedorn, B., Sletten, R. S. & Hallet, B. Sublimation and ice condensation in hyperarid soils: modeling results using field data from Victoria Valley, Antarctica. J. Geophys. Res. 112, F03017 (2007).
Dickinson, W. W. & Rosen, M. R. Antarctic permafrost: An analogue for water and diagenetic minerals on Mars. Geology 31, 199–202 (2003).
Nkem, J. N. et al. Salt tolerance and survival thresholds for two species of Antarctic soil nematodes. Polar Biol. 29, 643–651 (2006).
Adams, B. J. et al. Diversity and distribution of Victoria Land biota. Soil Biol. Biochem. 38, 3003–3018 (2006).
Freckman, D. W. & Virginia, R. A. Low-diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology 78, 363–369 (1997). This work established a conceptual model for delineating suitable and unsuitable soil habitats for invertebrates in the McMurdo Dry Valleys on the basis of abiotic properties.
Thomas, D. N. Photosynthetic microbes in freezing deserts. Trends Microbiol. 13, 87–88 (2005).
Friedmann, E. I. (ed.) Antarctic Microbiology (Wiley-Liss, New York, 1993). Although now somewhat out of date, this text still stands as a benchmark survey of the biology of Antarctic terrestrial and marine microbial ecology.
Novis, P. M. et al. Annual carbon fixation in terrestrial populations of Nostoc commune (Cyanobacteria) from an Antarctic dry valley is driven by temperature regime. Glob. Chang. Biol. 13, 1224–1237 (2007).
Matsumoto, G. I., Hirai, A., Hirota, K. & Watanuki, K. Organic geochemistry of the Mcmurdo Dry Valleys soil, Antarctica. Org. Geochem. 16, 781–791 (1990).
Burkins, M. B., Virginia, R. A., Chamberlain, C. P. & Wall, D. H. Origin and distribution of soil organic matter in Taylor Valley, Antarctica. Ecology 81, 2377–2391 (2000). This study shows that some Dry Valley soil food webs are heterotrophic, deriving their energy from external and, in some cases, ancient inputs of organic matter, whereas other food webs seem to have autotrophic bases.
Elberling, B. et al. Distribution and dynamics of soil organic matter in an Antarctic dry valley. Soil Biol. Biochem. 38, 3095–3106 (2006).
Hopkins, D. W. et al. Isotopic evidence for the provenance and turnover of organic carbon by soil microorganisms in the Antarctic dry valleys. Environ. Microbiol. 11, 597–608 (2009).
Hopkins, D. W. et al. Controls on the distribution of productivity and organic resources in Antarctic Dry Valley soils. Proc. Biol. Sci. 273, 2687–2695 (2006). An exceptional review of the effects of the abiotic factors that influence microbial activity in Dry Valley soils.
Cameron, R. E., King, J. & David, C. N. Soil toxocity in Antarctic Dry Valleys. Antarct. J. US 3, 164–166 (1968).
Horowitz, N. H. et al. Sterile soil from Antarctic organic analysis. Science 164, 1054–1056 (1969).
Benoit, R. E. & Hall, C. L. in Antarctic Ecology 697–701 (Academic, London, UK, 1970).
Cameron, R. E. in Antarctic Terrestrial Biology. Antarctic Research Series 195–260 (American Geophysical Union, Washington DC, 1972).
Cameron, R. E. & Ford, A. B. Baseline analysis of soils from the Pensacola Mountains. Antarct. J. US 9, 116–119 (1974).
Cameron, R. E., King, J. & David, C. N. in Antarctic Ecology 702–716 (Academic, London, UK, 1970).
Vishniac, W. V. & Mainzer, S. E. Soil microbiology studied in situ in the Dry Valleys of Antarctica. Antarct. J. US 7, 88–89 (1972).
Wynn-Williams, D. D. Ecological aspects of Antarctic microbiology. Adv. Microbial. Ecol. 11, 71–146 (1990).
Sjöling, S. & Cowan, D. A. High 16S rDNA bacterial diversity in glacial meltwater lake sediment, Bratina Island, Antarctica. Extremophiles 7, 275–282 (2003).
Ramsey, A. J. & Stannard, R. E. Numbers and viability of bacteria in ornothogenic soils of Antarctica. Polar Biol. 5, 195–198 (1986).
Buckley, D. H. & Schmidt, T. M. in Biodiversity of Microbial Life: Foundation of Earths Biosphere 183–208 (Wiley-Liss, New York, 2001).
DeLong, E. F. Microbial seascapes revisited. Curr. Opin. Microbiol. 4, 290–295 (2001).
Rappé, M. S. & Giovannoni, S. J. The uncultured microbial majority. Annu. Rev. Microbiol. 57, 369–394 (2003).
Johnson, R. M., Madden, J. M. & Swafford, J. R. in Terrestrial Biology III. Antarctic Research Series 35–64 (American Geophysical Union, Washington DC, 1978).
de la Torre, J. R., Goebel, B. M., Friedmann, E. I. & Pace, N. R. Microbial diversity of cryptoendolithic communities from the McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 69, 3858–3867 (2003). One of the first reports of the application of molecular phylogenetic methods to the study of microbial diversity in an Antarctic terrestrial system.
Aislabie, J. M., Jordan, S. & Barker, G. M. Relation between soil classification and bacterial diversity in soils of the Ross Sea region, Antarctica. Geoderma 144, 9–20 (2008).
Khan, N. Hypolithic Communities in the Miers Valley, Eastern Antarctica. Thesis, Univ. Western Cape, South Africa (2008).
Janssen, P. H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 72, 1719–1728 (2006).
Chanal, A. et al. The desert of Tataouine: an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria. Environ. Microbiol. 8, 514–525 (2006).
Babalola, O. O. et al. Phylogenetic analysis of actinobacterial populations associated with Antarctic Dry Valley mineral soils. Environ. Microbiol. 11, 566–576 (2009).
Yergeau, E. et al. Size and structure of bacterial, fungal and nematode communities along an Antarctic environmental gradient. FEMS Microbiol. Ecol. 59, 436–451 (2007).
Vestal, J. R. Carbon metabolism of the cryptoendolithic microbiota from the Antarctic desert. Appl. Environ. Microbiol. 54, 960–965 (1988).
Burkins, M. B., Virginia, R. A. & Wall, D. H. Organic carbon cycling in Taylor Valley, Antarctica: quantifying soil reservoirs and soil respiration. Glob. Chang. Biol. 7, 113–125 (2001). This is the first study to report estimates of soil respiration in the Dry Valleys and to consider ecosystem processes in the context of trophic dynamics.
Parsons, A. N., Barrett, J. E., Wall, D. H. & Virginia, R. A. Soil carbon dioxide flux in Antarctic dry valley ecosystems. Ecosystems 7, 286–295 (2004).
Hopkins, D. W. et al. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic dry valley. Soil Biol. Biochem. 38, 3130–3140 (2006). This paper provides insights into the environmental controls of microbial activity in Dry Valley soils.
Friedmann, E. I., Kappen, L., Meyer, M. A. & Nienow, J. A. Long-term productivity in the cryptoendolithic microbial community of the Ross Desert, Antarctica. Microb. Ecol. 25, 51–69 (1993). The most comprehensive contemporary paper that takes a functional approach to microbial communities in the Dry Valleys, with the first quantitative assessment of carbon cycling.
Howard-Williams, C., Hawes, I., Schwarz, A. M. & Hall, J. A. in Ecosystem Processes in Antarctic Ice-Free Landscapes (eds Lyons, W. B., Howard Williams, C. & Hawes, I.) 155–170 (Balkema, Rotterdam, 1997).
Raich, J. W. & Schlesinger, W. H. The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B Chem. Phys. Meteorol. 44, 81–99 (1992).
Brinkmann, M., Pearce, D. A., Convey, P. & Ott, S. The cyanobacterial community of polygon soils at an inland nunatuk. Polar Biol. 30, 1505–1511 (2007).
Wood, S. A., Rueckert, A., Cowan, D. A. & Cary, S. C. Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J. 2, 308–320 (2008).
Arenz, B. E., Held, B. W., Jurgens, J. A., Farrell, R. L. & Blanchette, R. A. Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil Biol. Biochem. 38, 3057–3064 (2006).
Cowan, D. A. & Ah Tow, L. Endangered Antarctic environments. Annu. Rev. Microbiol. 58, 649–690 (2004).
Schwarz, A. M. J., Green, J. D., Green, T. G. A. & Seppelt, R. D. Invertebrates associated with moss communities at Canada Glacier, Southern Victoria-Land, Antarctica. Polar Biol. 13, 157–162 (1993).
Lawley, B., Ripley, S., Bridge, P. & Convey, P. Molecular analysis of geographic patterns of eukaryotic diversity in Antarctic soils. Appl. Environ. Microbiol. 70, 5963–5972 (2004).
Vincent, W. F. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct. Sci. 12, 374–385 (2000).
Marshall, W. A. Biological particles over Antarctica. Nature 383, 680 (1996).
Russell, N. J. & Cowan, D. A. Handling of psychrophilic microorganisms. Extremophiles 35, 371–393 (2006).
Smith, M. C., Bowman, J. P., Scott, F. J. & Line, M. A. Sublithic bacteria associated with Antarctic quartz stones. Antarct. Sci. 12, 177–184 (2000).
Friedmann, E. I. Endolithic microorganisms in the Antarctic cold desert. Science 215, 1045–1053 (1982).
Solomon, S. Stratospheric ozone depletion: A review of concepts and history. Rev. Geophys. 37, 275–316 (1999).
Wynn-Williams, D. D., Edwards, H. G. M. & Garcia-Pichel, F. Functional biomolecules of Antarctic stromatolitic and endolithic cyanobacterial communities. Eur. J. Phycol. 34, 381–391 (1999).
Cockell, C. S. & Stokes, M. D. Ecology: widespread colonization by polar hypoliths. Nature 431, 414 (2004).
Friedmann, E. I., Hua, M. & Ocampo-Friedmann, R. Cryptoendolithic lichen and cyanobacterial communities in the Ross desert, Antarctica. Polarforschung 58, 251–259 (1988).
Siebert, J. et al. Cryptoendolithic microorganisms from Antarctic sandstone of linnaeus terrace (Asgard range): diversity, properties and interactions. Biodivers. Conserv. 5, 1337–1363 (1996).
Schlesinger, W. H. et al. Community composition and photosynthesis by photoautotrophs under quartz pebbles, southern Mojave Desert. Ecology 84, 3222–3231 (2003).
Dort, W. in Terrestrial Biology III. Antarctic research series 123–154 (American Geophysical Union, Washington DC, 1982).
Barwick, R. E. & Balham, R. W. Mummified seal carcasses in a deglaciated region of South Victoria Land, Antarctica. Tuatara 15, 165–180 (1967).
Dort, W. Mummified Seals of Southern Victoria Land. Antarct. J. US 5, 210–211 (1971).
Broeckert, W. A. & Olson, E. A. Lamont radiocarbon measurements VIII. Radiocarbon 3, 176–204 (1961).
Smith, C. R. & Baco, A. R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. 41, 311–354 (2003).
Turner, J. et al. Antarctic climate change during the last 50 years. Int. J. Climatol. 25, 279–294 (2005).
Chapman, W. L. & Walsh, J. E. A synthesis of Antarctic temperatures. J. Clim. 20, 4096–4117 (2007).
Steig, E. J. et al. Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature 457, 459–462 (2009); corrigendum 460, 766 (2009).
Doran, P. T. et al. Hydrologic response to extreme warm and cold summers in the McMurdo Dry Valleys, East Antarctica. Antarct. Sci. 20, 499–509 (2008).
Foreman, C., Wolf, C. F. & Priscu, J. C. Impact of episodic warming events on the physical, chemical and biological relationships of lakes in the McMurdo Dry Valleys, Antarctica. Aquatic Geochemistry 10, 239–268 (2004).
Esposito, R. M. M. et al. Antarctic climate cooling and response of diatoms in glacial meltwater streams. Geophys. Res. Lett. 33, L07406 (2006). This paper is an elegant analysis of the influence of climate variation on the relative abundance of endemic and cosmopolitan diatom species.
Ah Tow, L. & Cowan, D. A. Dissemination and survival of non-indigenous bacterial genomes in pristine Antarctic environments. Extremophiles 9, 385–389 (2005).
Hogg, I. D. et al. Biotic interactions in Antarctic terrestrial ecosystems: are they a factor? Soil Biol. Biochem. 38, 3035–3040 (2006).
Yergeau, E. et al. Environmental microarray analyses of Antarctic soil microbial communities. ISME J. 3, 340–351 (2009). An exciting contribution using cutting-edge microarray techniques to survey microbial communities in Antarctic soils.
Barrett, J. E. et al. Variation in biogeochemistry and soil biodiversity across spatial scales in a polar desert ecosystem. Ecology 85, 3105–3118 (2004).
Barrett, J. E. et al. Terrestrial ecosystem processes of Victoria Land, Antarctica. Soil Biol. Biochem. 38, 3019–3034 (2006).
Kennedy, A. D. Water as a limiting factor in the Antarctic terrestrial environment — a biogeographical synthesis. Arct. Alp. Res. 25, 308–315 (1993).
Treonis, A. M., Wall, D. H. & Virginia, R. A. Invertebrate biodiversity in Antarctic dry valley soils and sediments. Ecosystems 2, 482–492 (1999).
Ludwig, W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004).
Liles, M. R., Manske, B. F., Bintrim, S. B., Handelsman, J. & Goodman, R. M. A census of rRNA genes and linked genomic sequences within a soil metagenomic library. Appl. Environ. Microbiol. 69, 2684–2691 (2003).
Lipson, D. A. & Schmidt, S. K. Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl. Environ. Microbiol. 70, 2867–2879 (2004).
McCaig, A. E. et al. Nitrogen cycling and community structure of proteobacterial β-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl. Environ. Microbiol. 65, 213–220 (1999).
Sun, H. Y., Deng, S. P. & Raun, W. R. Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl. Environ. Microbiol. 70, 5868–5874 (2004).
Bate, D. B., Barrett, J. E., Poage, M. A. & Virginia, R. A. Soil phosphorus cycling in an Antarctic polar desert. Geoderma 144, 21–31 (2008).
Schade, J. D. & Hobbie, S. E. Spatial and temporal variation in islands of fertility in the Sonoran Desert. Biogeochemistry 73, 541–553 (2005).
Illeris, L., Michelsen, A. & Jonasson, S. Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 65, 15–29 (2003).
Acknowledgements
We acknowledge support from Antarctica New Zealand to S.C.C, I.R.M and D.A.C., from the Marsden Fund, New Zealand, to S.C.C. and I.R.M., from the Foundation of Research Science and Technology, New Zealand, to S.C.C., from the National Science Foundation, USA, to S.C.C. and J.E.B., and from the National Research Foundation, South Africa, to D.A.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Last glacial maximum
-
The time of maximum extent of the ice sheets during the last glaciation approximately 20,000 years ago.
- Biota
-
The total collection of organisms in a geographic region.
- Trophic level
-
An organism's position in the food chain.
- Nanutuks
-
Localized rocky, exposed, ice-free regions in terrestrial ice fields.
- Austral
-
Of the Southern Hemisphere.
- Sublime
-
Transition from the solid state to the gaseous phase with no intermediate liquid stage.
- Katabatic wind
-
A wind that carries high-density air from a higher elevation down a slope under the force of gravity. In the Antarctic, the build-up of high-density, cold air over the ice sheets and the elevation of the ice sheets bring into play enormous gravitational energy, propelling the winds at well over hurricane force.
- Permafrost layer
-
Soil that is at or below the freezing point of water (0 °C or 32 °F) for ≥2 years.
- Lithic environment
-
An environment that relates to or is composed of stone.
- Lacustrine sediments
-
Lake sediments
- Aeolian redistribution
-
Wind dispersal
- Ornithogenic
-
Derived from the deposition of the faecal matter of various bird species; ornithogenic material is a major source of nutrient input in the maritime Antarctic.
- Fell-field soil
-
Soil in an environment, usually alpine or tundra, where the dynamics of frost (freeze and thaw cycles) and of wind give rise to characteristic plant forms in scree interstices. In addition, the high porosity of the soil makes a fell-field a difficult place for plants to grow.
- Psychrophilic
-
Pertaining to an organism that prefers cold temperatures.
- Hypolithic
-
Pertaining to an organism that lives underneath rocks in climatically extreme deserts.
- Cryptoendolithic
-
Pertaining to an organism that colonizes structural cavities within porous rock.
- Photoautotrophic
-
Pertaining to an organism that is capable of synthesizing its own food using light.
- Heterotrophic
-
Pertaining to an organism that is capable of synthesizing its own food using chemical energy from inorganic substances.
- Mesic soil
-
Soil with a moderate or well-balanced supply of moisture.
- Psychrotrophic
-
Pertaining to an organism that is capable of surviving or even thriving in a cold environment.
- Mesophilic
-
Pertaining to an organism that grows best in moderate temperatures, typically between 15 °C and 40 °C (77 °F and 104 °F).
- Chasmolithic
-
Pertaining to an organism that colonizes fissures and cracks in the rock.
- Endolithic
-
Pertaining to an organism (archaeum, bacterium, fungus, lichen, alga or amoeba) that lives inside rock, coral or animal shells or in the pores between the mineral grains of a rock.
- Abiotic
-
Not associated with or derived from living organisms.
Rights and permissions
About this article
Cite this article
Cary, S., McDonald, I., Barrett, J. et al. On the rocks: the microbiology of Antarctic Dry Valley soils. Nat Rev Microbiol 8, 129–138 (2010). https://doi.org/10.1038/nrmicro2281
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2281
This article is cited by
-
Impact of meltwater flow intensity on the spatiotemporal heterogeneity of microbial mats in the McMurdo Dry Valleys, Antarctica
ISME Communications (2023)
-
Chlorine redox chemistry is widespread in microbiology
The ISME Journal (2023)
-
Native plant gardens support more microbial diversity and higher relative abundance of potentially beneficial taxa compared to adjacent turf grass lawns
Urban Ecosystems (2023)
-
Bringing Antarctica to the lab: a polar desert environmental chamber to study the response of Antarctic microbial communities to climate change
Polar Biology (2023)
-
Atmospheric chemosynthesis is phylogenetically and geographically widespread and contributes significantly to carbon fixation throughout cold deserts
The ISME Journal (2022)