Key Points
-
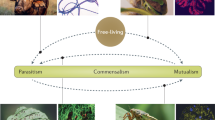
Symbiont transmission enables the host to maintain a symbiosis throughout generations.
-
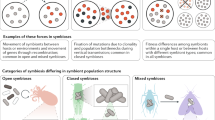
Two profoundly different strategies can be distinguished. The symbiont can be selected out of a pool of environmental bacteria (horizontal transmission), or the host offspring can take up the symbiont from the parents following a finely choreographed baton exchange (vertical transmission).
-
There are many variations of these two modes, and transmission can also involve both vertical and horizontal transfers and intraspecific or interspecific host switching.
-
From the moment of initial contact, the symbionts have a long journey to reach their final residence. This includes establishing contact with the host, entering the host, evading the host immune defences and travelling to the symbiont housing organ.
-
The two main transmission modes shape the evolution of the symbiotic partners.
Abstract
The perpetuation of symbioses through host generations relies on symbiont transmission. Horizontally transmitted symbionts are taken up from the environment anew by each host generation, and vertically transmitted symbionts are most often transferred through the female germ line. Mixed modes also exist. In this Review we describe the journey of symbionts from the initial contact to their final residence. We provide an overview of the molecular mechanisms that mediate symbiont attraction and accumulation, interpartner recognition and selection, as well as symbiont confrontation with the host immune system. We also discuss how the two main transmission modes shape the evolution of the symbiotic partners.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ewald, P. W. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 503, 295–306 (1987).
Yamamura, N. Vertical transmission and evolution of mutualism from parasitism. Theor. Popul. Biol. 44, 95–109 (1993).
Lipsitsch, M., Siller, S. & Nowak, M. A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50, 1729–1741 (1996).
Yamamura, N. Evolution of mutualistic symbiosis: a differential equation model. Res. Popul. Ecol. 38, 211–218 (1996).
Genkai-Kato, M. & Yamamura, N. Evolution of mutualistic symbiosis without vertical transmission. Theor. Popul. Biol. 55, 309–323 (1999).
Sharp, K. H., Eam, B., Faulkner, D. J. & Haygood, M. G. Vertical transmission of diverse microbes in the tropical sponge Corticium sp. Appl. Environ. Microbiol. 73, 622–629 (2007).
Steger, D. et al. Diversity and mode of transmission of ammonia-oxidizing archaea in marine sponges. Environ. Microbiol. 10, 1087–1094 (2008).
de Bary, A. Die Entstehung der Symbiose (Verlag von Karl, J. Trübner, Strassburg, 1879).
McFall-Ngai, M. J. Unseen forces: the influence of bacteria on animal development. Dev. Biol. 242, 1–14 (2002).
Hurek, T. & Reinhold-Hurek, B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J. Biotechnol. 106, 169–178 (2003).
Gage, D. J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68, 280–300 (2004).
Adams, D. G., Bergman, B., Nierzwicki-Bauer, S. A., Rai, A. N. & Schüßler, A. in The Prokaryotes 331–363 (Springer, New York, 2006). This book chapter provides an excellent overview of plant–cyanobacteria symbioses.
Jones, K. J., Kobayashi, H., Davies, B. W., Taga, M. E. & Walker, G. C. How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nature Rev. Microbiol. 5, 619–633 (2007). An excellent review of legume–rhizobia symbiosis establishment.
Usher, K. M., Bergman, B. & Raven, J. A. Exploring cyanobacterial mutualisms. 38, 255–273 (2007).
Adams, D. G. & Duggan, P. S. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 59, 1047–1058 (2008).
Oldroyd, G. E. D. & Downie, J. A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 59, 519–546 (2008).
Taylor, M. W., Radax, R., Steger, D. & Wagner, M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 71, 295–347 (2007).
Usher, K. M. The ecology and phylogeny of cyanobacterial symbionts in sponges. Mar. Ecol. 29, 178–192 (2008).
Dubilier, N., Bergin, C. & Lott, C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Rev. Microbiol. 6, 725–740 (2008). A scrupulous account of marine chemosynthetic symbioses.
Vrijenhoek, R. C. in Topics in Geobiology. The Vent and Sea Biota. (ed. Kiel, S.) (Springer, Berlin, Germany) (in the press).
Herbert, E. E. & Goodrich-Blair, H. Friend and foe: the two faces of Xenorhabdus nematophila. Nature Rev. Microbiol. 5, 634–646 (2007). An outstanding summary of the Steinernema – Xenorhabdus symbiosis.
Clarke, D. J. Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell. Microbiol. 10, 2159–2167 (2008).
Bright, M. & Giere, O. Microbial symbiosis in Annelida. Symbiosis 38, 1–45 (2005).
Graf, J., Kikuchi, Y. & Rio, R. V. M. Leeches and their microbiota: naturally simple symbiosis models. Trends Microbiol. 14, 365–371 (2006).
Buchner, P. Endosymbiosis of Animals with Plant Microorganisms (Wiley & Sons, New York, 1965). A seminal and exhaustive book on insect symbioses.
Douglas, A. E. Mycetocyte symbiosis in insects. Biol. Rev. Camb. Philos. Soc. 64, 409–434 (1989). This is a fine guide to the overwhelming plethora of transmission modes of insect symbionts.
Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59, 155–189 (2005).
Dale, C. & Moran, N. A. Molecular interactions between bacterial symbionts and their hosts. Cell 126, 453–465 (2006).
Moran, N. A., McCutcheon, J. P. & Nakabachi, A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190 (2008).
Moya, A., Peretó, J., Gil, R. & Latorre, A. Learning how to live together: genomic insights into prokaryote–animal symbioses. Nature Rev. Genet. 9, 218–229 (2008).
Nyholm, S. V. & McFall-Ngai, M.J. The winnowing: establishing the squid–Vibrio symbiosis. Nature Rev. Microbiol. 2, 632–642 (2004). A compelling overview of the establishment of the squid– Vibrio spp. symbiosis.
Visick, K. L. & Ruby, E. G. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 9, 632–638 (2006).
Hooper, L. V. & Gordon, J. I. Commensal host-bacterial relationships in the gut. Science 292, 1115–1118 (2001).
Sonnenburg, J. L., Angenent, L. T. & Gordon, J. I. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nature Immunol. 5, 569–573 (2004).
Cheesman, S. E. & Guillemin, K. We know you are in there: conversing with the indigenous gut microbiota. Res. Microbiol. 158, 2–9 (2007). This review ponders the parallels between vertebrate microbial mutualisms and the squid– Vibrio spp. symbiosis.
Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R. & Gordon, J. I. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Rev. Microbiol. 6, 776–788 (2008).
West, N. & Adams, D. G. Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Appl. Environ. Microbiol. 63, 4479–4484 (1997).
Costa, J. L., Paulsrud, P., Rikkinen, J. & Lindblad, P. Genetic diversity of Nostoc symbionts endophytically associated with two bryophyte species. Appl. Environ. Microbiol. 67, 4393–4396 (2001).
Kikuchi, Y., Hosokawa, T. & Fukatsu, T. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 73, 4308–4316 (2007). The first report of a horizontally transmitted insect symbiont.
Lee, K.-H. & Ruby, E. G. Detection of the light organ symbiont, Vibrio fischeri, in hawaiian seawater by using lux gene probes. Appl. Environ. Microbiol. 58, 942–947 (1992).
Gros, O., Liberge, M., Heddi, A., Khatchadourian, C. & Felbeck, H. Detection of the free-living forms of sulfide-oxidizing gill endosymbionts in the lucinid habitat (Thalassia testdinum environment). Appl. Environ. Microbiol. 69, 6264–6267 (2003).
Aida, M. et al. Distribution and population of free-living cells related to endosymbiont A harboured in Oligobrachia mashikoi (a siboglinid polychaete) inhabiting Tsukumo Bay. Microbes Environ. 23, 81–88 (2008).
Harmer, T. L. et al. Free-living tube worm endosymbionts found at deep-sea vents. Appl. Environ. Microbiol. 74, 3895–3898 (2008).
Kiers, E. T., Rousseau, R. A., West, S. A. & Denison, R. F. Host sanctions and the legume-rhizobium mutualism. Nature 425, 78–81 (2003).
Salerno, J. L. et al. Characterization of symbiont populations in life-history stages of mussels from chemosynthetic environments. Biol. Bull. 208, 145–155 (2005).
Callsen-Cencic, P. & Flügel, H. J. Larval development and the formation of the gut of Siboglinum poseidoni Flügel & Langhof (Pogonophora, Perviata). Evidence of protostomian affinity. Sarsia 80, 73–89 (1995).
McCann, J., Stabb, E. V., Milikan, D. S. & Ruby, E. G. Population effects of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 69, 5928–5934 (2003).
Gros, O., Frenkiel, L. & Moueza, M. Gill filament differentiation and experimental colonization by symbiotic bacteria in aposymbiotic juveniles of Codakia orbicuaris (Bivalvia: Lucinidae). Invertebr. Reprod. Dev. 34, 219–231 (1998).
Nussbaumer, A. D., Fisher, C. R. & Bright, M. Horizontal endosymbiont transmission in hydrothermal vent tubeworms. Nature 441, 345–348 (2006). A groundbreaking study of the acquisition of vestimentiferan symbionts.
Bates, J. M. et al. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 297, 374–386 (2006).
Cooper, J. E. Multiple responses of rhizobia to flavonoids during legume root infection. Adv. Bot. Res. 41, 1–62 (2004).
Brencic, A. & Winans, S. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol. Mol. Biol. Rev. 69, 155–194 (2005).
Robidart, J. C. et al. Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ. Microbiol. 10, 727–737 (2008).
Gros, O., Darrasse, A., Durand, P., Frenkiel, L. & Moueza, M. Environmental transmission of a sulfur-oxidizing bacterial gill endosymbiont in the tropical lucinid bivalve Codakia orbicularis. Appl. Environ. Microbiol. 62, 2324–2330 (1996).
Ruby, E. G. & Lee, K.-H. The Vibrio fischeri-Euprymna scolopes light orga. association: current ecological paradigms. Appl. Environ. Microbiol. 64, 805–812 (1998).
Stabb, E. V. & Ruby, E. G. Contribution of pilA to competitive colonization of the squid Euprymna scolopes by Vibrio fischeri. Appl. Environ. Microbiol. 69, 820–826 (2003).
Adin, D. M. et al. Characterization of htrB and msbB mutants of the light organ symbiont Vibrio fischeri. Appl. Environ. Microbiol. 74, 633–644 (2007).
Fauvart, M. & Michiels, J. Rhizobial secreted proteins as determinants of host specificity in the rhizobium-legume symbiosis. FEMS Microbiol. Lett. 285, 1–9 (2008).
Aeckersberg, F., Lupp, C., Feliciano, B. & Ruby, E. G. Vibrio fischeri outer membrane protein OmpU plays a role in normal symbiotic colonization. J. Bacteriol. 183, 6590–6597 (2001).
Xu, J. et al. A genomic view of the human–Bacteroides thetaiotaomicron symbiosis. Science 299, 2074–2076 (2003).
Chun, C. K. et al. Effects of colonization, luminescence, and autoinducer on host transcription during development of the squid-vibrio association. Proc. Natl Acad. Sci. USA. 105, 11323–11328 (2008).
Bulgheresi, S., Schabussova, I., Mullin, N. P., Maizels, R. M. & Ott, J. A. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 72, 2950–2956 (2006).
Gourdine, J. P. & Smith-Ravin, E. J. Analysis of a cDNA-derived sequence of a novel mannose-binding lectin, codakine, from the tropical clam Codakia orbicularis. Fish Shellfish Immunol. 22, 498–509 (2007).
Yip, E. S., Grublesky, B. T., Hussa, E. A. & Visick, K. L. A novel, conserved cluster of genes promotes symbiotic colonization and σ54-dependent biofilm formation by Vibrio fischeri. Mol. Microbiol. 57, 1485–1498 (2005).
Hussa, E. A., O'Shea, T. M., Darnell, C. L., Ruby, E. G. & Visick, K. L. Two-component response regulators of Vibrio fischeri: identification, mutagenesis, and characterization. J. Bacteriol. 189, 5825–5838 (2007).
Mandel, M. J., Wollenberg, M. S., Stabb, E. V., Visick, K. L. & Ruby, E. G. A single regulatory gene is sufficient to alter bacterial host range. Nature 458, 215–218 (2009).
Hoang, H. H., Becker, A. & Gonzalez, J. E. The LuxR homolog ExpR, in combination with the Sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J. Bacteriol. 186, 5460–5472 (2004).
Gao, M. et al. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 187, 7931–7944 (2005).
Cooper, J. E. Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J. Appl. Microbiol. 103, 1355–1365 (2007).
De Hoff, P. L., Brill, L. M. & Hirsch, A. M. Plant lectins: the ties that bind in root symbiosis and plant defense. Mol. Genet. Genomics 282, 1–15 (2009).
Fujishige, N. A. et al. Rhizobium common nod genes are required for biofilm formation. Mol. Microbiol. 67, 504–515 (2008).
Goormachtig, S., Capoen, W. & Holsters, M. Rhizobium infection: lessons from the versatile nodulation behaviour of water-tolerant legumes. Trends Plant Sci. 9, 518–522 (2004).
Bartsev, A. et al. NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol. 134, 871–879 (2004).
Davidson, S. K., Koropatnick, T. A., Kossmehl, R., Sycuro, L. & McFall-Ngai, M. J. NO means 'yes' in the squid-vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 6, 1139–1151 (2004).
Moran, N. A. & Dunbar, H. E. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl Acad. Sci. USA 103, 12803–12806 (2006). This is the first report of intraspecific symbiont transfer.
Usher, K. M., Sutton, D. C., Toze, S., Kuo, J. & Fromont, J. Inter-generational transmission of microbial symbionts in the marine sponge Chondrilla australiensis (Demospongiae). Mar. Freshw. Res. 56, 125–131 (2005).
Serbus, L. R., Casper-Lindley, C., Landmann, F. & Sullivan, W. The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42, 1–25 (2008). A must-read review on Wolbachia spp. transmission and interactions with its host.
Perkins, S. K. & Peters, G. A. The Azolla-Anabaena symbiosis: endophyte continuity in the Azolla life-cycle is facilitated by epidermal trichomes. I. Partitioning of the endophytic Anabaena into developing sporocarps. New Phytol. 123, 53–64 (1993).
Werren, J. H., Skinner, S. W., Huger, A. M. Male-killing bacteria in a parasitic wasp. Science 231, 990–992 (1986).
Huigens, M. E. et al. Infectious parthenogenesis. Nature 405, 178–179 (2000).
Huigens, M. E., de Almeida, R. P., Boons, P. A., Luck, R. F. & Stouthamer, R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. Biol. Sci. 271, 509–515 (2004).
Chiel, E. et al. Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS ONE 4, e4767 (2009).
Brennan, L. J., Keddie, B. A., Braig, H. R. & Harris, H. L. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS ONE 3, e2083 (2008).
Frank, S. A. Host control of symbiont transmission: the separation of symbionts into germ and soma. Am. Nat. 148, 1113–1124 (1996).
Dale, C., Plague, G. R., Wang, B., Ochman, H. & Moran, N. A. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc. Natl Acad. Sci. USA 99, 12397–12402 (2002).
Giere, O. & Langheld, C. Structural organisation, transfer and biological fate of endosymbiotic bacteria in gutless oligochaetes. Mar. Biol. 93, 641–650 (1987).
Hirose, E. Plant rake and algal pouch of the larvae in the tropical ascidian Diplosoma similis: an adaptation for vertical transmission of photosynthetic symbionts Prochloron sp. Zool. Sci. 17, 233–240 (2000). An exquisite morphological study of ascidian adaptations to symbiont vertical transmission.
Hirose, E. & Fukuda, T. Vertical transmission of photosymbionts in the colonial ascidian Didemnum molle: the larval tunic prevents symbionts from attaching to the anterior part of larvae. Zool. Sci. 23, 669–674 (2006).
Hirose, E., Adachi, R. & Kuze, K. Sexual reproduction of the Prochloron-bearing ascidians, Trididemnum cyclops and Lissoclinum bistratum, in subtropical waters: seasonality and vertical transmission of photosymbionts. J. Mar. Biolog. Assoc. UK 86, 175–179 (2006).
Hirose, E. & Hirose, M. Morphological process of vertical transmission of photosymbionts in the colonial ascidian Trididemnum miniatum Kott, 1977. Mar. Biol. 150, 359–367 (2007).
Ereskovsky, A. V. & Boury-Esnault, N. Cleavage pattern in Oscarella species (Porifera, Demospongiae, Homoscleromorpha): transmission of maternal cells and symbiotic bacteria. J. Nat. Hist. 36, 1761–1775 (2002).
Kaye, H. R. Sexual reproduction in four Caribbean commercial sponges. II. Oogenesis and transfer of bacterial symbionts. Invertebr. Reprod. Dev. 19, 13–24 (1991).
Ereskovsky, A. V., Gonobobleva, E. & Vishnyakov, A. Morphological evidence for vertical transmission of symbiotic bacteria in the viviparous sponge Halisarca dujardini Johnston (Porifera, Demospongiae, Halisarcida). Mar. Biol. 146, 869–875 (2005).
Sharp, K. H., Davidson, S. K. & Haygood, M. G. Localization of 'Candidatus Endobugula sertula' and the bryostatins throughout the life cycle of the bryozoan Bugula neritina. ISME J. 1, 693–702 (2007). A first-rate description of the transmission of a bryozoan symbiont.
Cary, S. C. Vertical transmission of a chemoautotrophic symbiont in the protobranch bivalve, Solemya reidi. Mol. Marine Biol. Biotechnol. 3, 121–130 (1994).
Cary, S. C. & Giovannoni, S. J. Transovarial inheritance of endosymbiotic bacteria in clams inhabiting deep-sea hydrothermal vents and cold seeps. Proc. Natl Acad. Sci. USA 90, 5695–5699 (1993).
Endow, K. & Ohta, S. Occurrence of bacteria in the primary oocytes of vesicomyid clam Calyptogena soyoae. Mar. Ecol. Prog. Ser. 64, 309–311 (1990).
Eberle, M. W. & McLean, D. L. Initiation and orientation of the symbiote migration in the human body louse Pediculus humanus L. J. Insect Physiol. 28, 417–422 (1982).
Eberle, M. W. & McLean, D. L. Observation of symbiote migration in human body lice with scanning and transmission electron microscopy. Can. J. Microbiol. 29, 755–762 (1983). This paper provides some marvellous micrographs of the louse symbionts on their way to the ovaries.
Sasaki-Fukatsu, K. et al. Symbiotic bacteria associated with stomach discs of human lice. Appl. Environ. Microbiol. 72, 7349–7352 (2006).
Ciche, T. A., Kim, K.-S. & Kaufmann-Daszczuk, B. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 74, 2275–2287 (2008). An accurate description of a peculiar and unexpected vertical transmission route.
Brugirard-Ricaud, K. et al. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 7, 363–371 (2005).
Martens, E. C. & Goodrich-Blair, H. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell. Microbiol. 7, 1723–1735 (2005).
Rio, R. V., Maltz, M., McCormick, B., Reiss, A. & Graf, J. Symbiont succession during the embryonic development of the european medicinal leech, Hirudo verbana. Appl. Environ. Microbiol. 5, 6890–6895 (2009).
Büsing, K.-H., Döll, W. & Freytag, K. Die Bakterienflora der medizinischen medizinischen Blutegel. Arch. Mikrobiol. 19, 52–86 (1953).
Silver, A. C. et al. Interaction between innate immune cells and a bacterial type III secretion system in mutualistic and pathogenic associations. Proc. Natl Acad. Sci. USA 104, 9481–9486 (2007).
Usher, K. M., Kuo, J., Fromont, J. & Sutton, D. C. Vertical transmission of cyanobacterial symbionts in the marine sponge Chondrilla australiensis (Demospongiae). Hydrobiologia 461, 15–23 (2001).
Gottlieb, Y. et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 22, 2591–2599 (2008).
Miura, T. et al. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. 295B, 59–81 (2003).
Mira, A. & Moran, N. A. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44, 137–143 (2002).
Cowles, C. E. & Goodrich-Blair, H. The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema carpocapsae nematodes. J. Bacteriol. 190, 4121–4128 (2008).
Chandra, H., Khandelwal, P., Khattrl, A. & Banerjee, N. Type 1 fimbriae of insecticidal bacterium Xenorhabdus nematophila is necessary for growth and colonization of its symbiotic host nematode Steinernema carpocapsiae. Environ. Microbiol. 10, 1285–1295 (2008).
Davidson, S. K. & Stahl, D. A. Selective recruitment of bacteria during embryogenesis of an earthworm. ISME J. 2, 510–518 (2008). This is a high quality account of the symbiont journey to the nephridia of developing earthworms.
Gustafson, R. G. & Reid, R. G. B. Larval and post-larval morphogenesis in the gutless protobranch bivalve Solemya reidi (Cryptodonta: Solemyidae). Mar. Biol. 97, 373–387 (1988).
Gustafson, R. G. & Reid, R. G. B. Association of bacteria with larvae of the gutless protobranch bivalve Solemya reidi (Cryptodonta: Solemyidae). Mar. Biol. 97, 389–401 (1988).
Ohta, T. Slightly deleterious mutant substitutions in evolution. Nature 246, 96–98 (1973).
Moran, N. A. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl Acad. Sci. USA 93, 2873–2878 (1996).
Funk, D. J., Wernegreen, J. J. & Moran, N. A. Intraspecific variation in symbiont genomes: bottlenecks and the Aphid-Buchnera association. Genetics 157, 477–489 (2001).
Shigenobu, S., Watanabe, H., Hattori, M., Sakaki, Y. & Ishikawa, H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407, 81–86 (2000).
Rio, R. V. M., Lefevre, C., Heddi, A. & Aksoy, S. Comparative genomics of insect-symbiotic bacteria: influence of host environment on microbial genome composition. Appl. Environ. Microbiol. 69, 6825–6832 (2003).
Woyke, T. et al. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature 443, 950–955 (2006).
Charles, H., Heddi, A., Guillaud, J., Nardon, C. & Nardon, P. A molecular aspect of symbiotic interactions between the weevil Sitophilus oryzae and its endosymbiotic bacteria: over-expression of a chaperonin. Biochem. Biophys. Res. 239, 769–774 (1997).
Plague, G. R., Dunbar, H. E., Tran, P. L. & Moran, N. A. Extensive proliferation of transposable elements in heritable bacterial symbionts. J. Bacteriol. 190, 777–779 (2008).
Mira, A., Ochman, H. & Moran, N. A. Deletional bias and the evolution of bacterial genomes. Trends Genet. 17, 589–596 (2001).
Moran, N. A. & Plague, G. R. Genomic changes following host restriction in bacteria. Curr. Opin. Genet. Dev. 14, 627–633 (2004).
Gros, O., Liberge, M. & Felbeck, H. Interspecific infection of aposymbiotic juveniles of Codakia orbicularis by various tropical lucinid gill-endosymbionts. Mar. Biol. 142, 57–66 (2003).
Kikuchi, Y., Meng, X. Y. & Fukatsu, T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71, 4035–4043 (2005).
Nishiguchi, M. K., Ruby, E. G. & McFall-Ngai, M. J. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-Vibrio symbioses. Appl. Environ. Microbiol. 64, 3209–3213 (1998).
Won, Y. - J., Jones, W. J. & Vrijenhoek, R. C. Absence of cospeciation between deep-sea mytilids and their thiotrophic endosymbionts. J. Shellfish Res. 27, 129–138 (2008).
Goffredi, S. K., Hurtado, L. A., Hallam, S. & Vrijenhoek, R. C. Evolutionary relationships of deep-sea vent and cold seep clams (Mollusca: Vesicomyidae) of the “pacifica/lepta” species complex. Mar. Biol. 142, 311–320 (2003).
Hurtado, L. A., Mateos, M., Lutz, R. A. & Vrijenhoek, R. C. Coupling of bacterial endosymbiont and host mitochondrial genomes in the hydrothermal vent clam Calyptogena magnifica. Appl. Environ. Microbiol. 69, 2058–2064 (2003).
Stewart, F. J., Young, C. R. & Cavanaugh, C. M. Lateral symbiont acquisition in a maternally transmitted chemosynthetic clam endosymbiosis. Mol. Biol. Evol. 25, 673–687 (2008).
Allen, J. M., Reed, D. L., Perotti, M. A. & Braig, H. R. Evolutionary relationships of “Candidatus Riesia spp.”, endosymbiotic Enterobacteriaceae living within hematophagous primate lice. Appl. Environ. Microbiol. 73, 1659–1664 (2007).
Schramm, A. et al. Acidovorax-like symbionts in the nephridia of earthworms. Environ. Microbiol. 5, 804–809 (2003).
Shoemaker, D. D. et al. The distribution of Wolbachia in fig wasps: correlations with host phylogeny, ecology and population structure. Proc. R. Soc. Lond., B, Biol. Sci. 269, 2257–2267 (2002).
Kikuchi, Y. & Fukatsu, T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl. Environ. Microbiol. 69, 6082–6090 (2003).
Reuter, M. & Keller, L. High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta. Mol. Biol. Evol. 20, 748–753 (2003).
Münchhoff, J. et al. Host specificity and phylogeography of the prochlorophyte Prochloron sp., an obligate symbiont in didemnid ascidians. Environ. Microbiol. 9, 890–899 (2007).
Blazejak, A., Kuever, J., Erseus, C., Amann, R. & Dubilier, N. Phylogeny of 16S rRNA, ribulose 1, 5-bisphosphate carboxylase/oxygenase, and adenosine 5′-phosphosulfate reductase genes from gamma- and alphaproteobacterial symbionts in gutless marine worms (Oligochaeta) from Bermuda and the Bahamas. Appl. Environ. Microbiol. 72, 5527–5536 (2006).
Lim-Fong, G. E., Regali, L. A. & Haygood, M. G. Evolutionary relationships of “Candidatus Endobugula” bacterial symbionts and their Bugula bryozoan hosts. Appl. Environ. Microbiol. 72, 3605–3609 (2008).
Krueger, D. M., Gustafson, R. G. & Cavanaugh, C. M. Vertical transmission of chemoautotrophic symbionts in the bivalve Solemya velum (Bivalvia: Protobranchia). Biol. Bull. 190, 195–202 (1996).
Schmitt, S., Angermeier, H., Schiller, R., Lindquist, N. & Hentschel, U. Molecular microbial diversity survey of sponge reproductive stages and mechanistic insights into vertical transmission of microbial symbionts. Appl. Environ. Microbiol. 74, 7694–7708 (2008).
Davidson, S. K. & Stahl, D. A. Transmission of nephridial bacteria of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 72, 769–775 (2006).
Perotti, M. A., Allen, J. M., Reed, D. L. & Braig, H. R. Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J. 21, 1058–1066 (2007).
Nardon, P. Contribution à l'ètude des symbiotes ovaries de Sitophilus sasakii: localisation, histochimie et ultrastructure chez la femelle adults. C. R. Acad. Sci. III, Sci. 272, 2975–2978 (1971).
Meeks, J. C. et al. An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosyn. Res. 70, 85–106 (2001).
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) grant P20282-B17 (M.B.) and the Austrian Research Promotion Agency (FFG) grant 814324 (S.B.). We particularly thank D. Distel, H. Felbeck, H. Goodrich-Blair, Y. Gottlieb, J. Graf, A.Heddi, U. Hentschel, J. A. Ott, Robert C. Vrijenhoek and W. Miller for insightful discussions and valuable comments on the manuscript, and S. Davidson, M. Haygood, E. Hirose, M. J. McFall-Ngai and K. Sharp for their help with some figures. We also thank S. Espada Hinojosa and I. Kolar for their assistance with the literature and M. Stachowitsch for editorial work. Several original papers on transmission were not cited owing to space limitations.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
(PDF 443 kb)
Related links
Related links
DATABASES
Entrez Genome Project
Candidatus Endoriftia persephone
Candidatus Hamiltonella defensa
Candidatus Regiella insecticola
Candidatus Riesia pediculicola
Candidatus Serratia symbiotica
FURTHER INFORMATION
Glossary
- Pelagic dispersal
-
The spreading of an organism, usually its larval stage, in the water column.
- Sporophyte
-
The diploid generation in the life cycle of the hornwort, which develops from the zygote and produces spores.
- Thallus
-
Undifferentiated vegetative tissue in hornworts that has a circular or ribbon-like arrangement.
- Hormogonium
-
A short, motile filament that lacks heterocysts. Hormogonia provide a means of dispersal for otherwise immotile cyanobacteria.
- Flavonoid
-
A 2-phenyl-1,4-benzopyrone derivative, produced by plants, that serves as a defence and signalling compound.
- Swarming motility
-
Rapid and coordinated movement of a bacterial population across solid or semi-solid surfaces that is powered by numerous flagella.
- Indeterminate nodule
-
A nodule formed by plants of some clades of legumes that develops a continuously growing nodule meristem at the distal end and has zones of tissue at different stages of development.
- Trophosome
-
Symbiont-housing organ in siboglinid tubeworms, of mesodermal origin in Vestimentifera, and endodermal origin in Frenulata.
- Symbiosome
-
Host membrane surrounding the symbionts.
- Nurse cell
-
A polytenic germline cell in insects that contributes to the development of the oocyte, producing the bulk of its cytoplasm and multiple nuclei.
- Bacteriome
-
A specialized organ containing host cells (bacteriocytes), which house endosymbiotic bacteria.
- Ontogenetic stage
-
Life cycle phases in the development from the fertilized egg to the adult.
- Mesohyl
-
A proteinaceous gelatinous matrix between the epidermis and gastrodermis of cnidarians.
- Haemocoel
-
The space between the organs through which haemolymph circulates in arthropods.
- Parthenogenetic egg
-
An unfertilized egg that develops into a new individual.
Rights and permissions
About this article
Cite this article
Bright, M., Bulgheresi, S. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol 8, 218–230 (2010). https://doi.org/10.1038/nrmicro2262
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2262
This article is cited by
-
Genesis of ectosymbiotic features based on commensalistic syntrophy
Scientific Reports (2024)
-
Morphological aspects of oogenesis in the deep-sea clam Calyptogena pacifica (Pliocardiinae; Vesicomyidae)
Marine Biology (2024)
-
An improved transmissibility model to detect transgenerational transmitted environmental effects
Genetics Selection Evolution (2023)
-
Effects of host species on microbiota composition in Phlebotomus and Lutzomyia sand flies
Parasites & Vectors (2023)
-
New insight into the bark beetle ips typographus bacteriome reveals unexplored diversity potentially beneficial to the host
Environmental Microbiome (2023)