Key Points
-
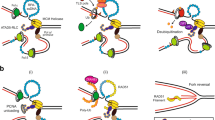
The sliding clamp proliferating cell nuclear antigen (PCNA) has a crucial role as a processivity factor for DNA replication in eukaryotic cells. PCNA provides a central platform for coordinating many replication-associated processes, such as DNA damage repair or bypass, chromatin establishment and sister chromatid cohesion.
-
A large number of cellular proteins compete for binding to a common surface on PCNA, necessitating tight, multilayered regulatory mechanisms that enable a fine-tuned interplay between PCNA and appropriate partner proteins at different stages of DNA replication and associated processes.
-
The PCNA-interacting protein (PIP) box, which is present in numerous proteins, is an important determinant for PCNA binding. Relative PCNA-binding affinities of PIP boxes establish a basic hierarchy of PCNA interactions.
-
Regulatory mechanisms controlling and modulating PCNA–protein interactions include post-translational modifications of PCNA and its associated proteins, accessory factors regulating PCNA–protein interactions and selective proteasome-dependent degradation of PCNA-bound proteins.
-
Mono- and polyubiquitylation of PCNA have key roles in enabling bypass of replication-associated DNA damage via translesion DNA synthesis and template switching, respectively.
-
Many new PCNA-binding proteins have been identified and characterized in recent years, broadening our understanding of the organization and regulation of PCNA-dependent processes underlying genome stability maintenance at the replication fork.
Abstract
Proliferating cell nuclear antigen (PCNA) has a central role in promoting faithful DNA replication, providing a molecular platform that facilitates the myriad protein–protein and protein–DNA interactions that occur at the replication fork. Numerous PCNA-associated proteins compete for binding to a common surface on PCNA; hence these interactions need to be tightly regulated and coordinated to ensure proper chromosome replication and integrity. Control of PCNA–protein interactions is multilayered and involves post-translational modifications, in particular ubiquitylation, accessory factors and regulated degradation of PCNA-associated proteins. This regulatory framework allows cells to maintain a fine-tuned balance between replication fidelity and processivity in response to DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010).
Moldovan, G. L., Pfander, B. & Jentsch, S. PCNA, the maestro of the replication fork. Cell 129, 665–679 (2007).
Indiani, C. & O'Donnell, M. The replication clamp-loading machine at work in the three domains of life. Nature Rev. Mol. Cell Biol. 7, 751–761 (2006).
Kelch, B. A., Makino, D. L., O'Donnell, M. & Kuriyan, J. How a DNA polymerase clamp loader opens a sliding clamp. Science 334, 1675–1680 (2011).
Rauen, M., Burtelow, M. A., Dufault, V. M. & Karnitz, L. M. The human checkpoint protein hRad17 interacts with the PCNA-like proteins hRad1, hHus1, and hRad9. J. Biol. Chem. 275, 29767–29771 (2000).
Zou, L., Cortez, D. & Elledge, S. J. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16, 198–208 (2002).
Mayer, M. L., Gygi, S. P., Aebersold, R. & Hieter, P. Identification of RFC(Ctf18p, Ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol. Cell 7, 959–970 (2001).
Ogi, T. et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol. Cell 37, 714–727 (2010).
Crabbe, L. et al. Analysis of replication profiles reveals key role of RFC–Ctf18 in yeast replication stress response. Nature Struct. Mol. Biol. 17, 1391–1397 (2010).
Lee, K. Y., Fu, H., Aladjem, M. I. & Myung, K. ATAD5 regulates the lifespan of DNA replication factories by modulating PCNA level on the chromatin. J. Cell Biol. 200, 31–44 (2013).
Johnson, A. & O'Donnell, M. Cellular DNA replicases: components and dynamics at the replication fork. Annu. Rev. Biochem. 74, 283–315 (2005).
Kong, X. P., Onrust, R., O'Donnell, M. & Kuriyan, J. Three-dimensional structure of the β-subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell 69, 425–437 (1992).
Krishna, T. S., Kong, X. P., Gary, S., Burgers, P. M. & Kuriyan, J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell 79, 1233–1243 (1994).
Gulbis, J. M., Kelman, Z., Hurwitz, J., O'Donnell, M. & Kuriyan, J. Structure of the C-terminal region of p21WAF1/CIP1 complexed with human PCNA. Cell 87, 297–306 (1996). References 13 and 14 describe the crystal structure of yeast PCNA and the structural basis of the p21CIP1/WAF1 PIP box binding to PCNA.
Ivanov, I., Chapados, B. R., McCammon, J. A. & Tainer, J. A. Proliferating cell nuclear antigen loaded onto double-stranded DNA: dynamics, minor groove interactions and functional implications. Nucleic Acids Res. 34, 6023–6033 (2006).
Mayanagi, K. et al. Architecture of the DNA polymerase B–proliferating cell nuclear antigen (PCNA)–DNA ternary complex. Proc. Natl Acad. Sci. USA 108, 1845–1849 (2011).
Georgescu, R. E. et al. Structure of a sliding clamp on DNA. Cell 132, 43–54 (2008).
Xu, H., Zhang, P., Liu, L. & Lee, M. Y. A novel PCNA-binding motif identified by the panning of a random peptide display library. Biochemistry 40, 4512–4520 (2001).
Bruning, J. B. & Shamoo, Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-δ p66 subunit and flap endonuclease-1. Structure 12, 2209–2219 (2004).
Fridman, Y. et al. Subtle alterations in PCNA-partner interactions severely impair DNA replication and repair. PLoS Biol. 8, e1000507 (2010).
Dionne, I., Nookala, R. K., Jackson, S. P., Doherty, A. J. & Bell, S. D. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol. Cell 11, 275–282 (2003).
Sakurai, S. et al. Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J. 24, 683–693 (2005).
Xing, G., Kirouac, K., Shin, Y. J., Bell, S. D. & Ling, H. Structural insight into recruitment of translesion DNA polymerase Dpo4 to sliding clamp PCNA. Mol. Microbiol. 71, 678–691 (2009).
Bubeck, D. et al. PCNA directs type 2 RNase H activity on DNA replication and repair substrates. Nucleic Acids Res. 39, 3652–3666 (2011).
Mayanagi, K. et al. Mechanism of replication machinery assembly as revealed by the DNA ligase–PCNA–DNA complex architecture. Proc. Natl Acad. Sci. USA 106, 4647–4652 (2009).
Gilljam, K. M. et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J. Cell Biol. 186, 645–654 (2009).
Ciccia, A. et al. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Mol. Cell 47, 396–409 (2012).
Ulrich, H. D. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair (Amst.) 8, 461–469 (2009).
Hoege, C., Pfander, B., Moldovan, G. L., Pyrowolakis, G. & Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 (2002). Discovery that PCNA is modified by ubiquitylation in response to DNA damage and by SUMOylation during an unperturbed S phase.
Stelter, P. & Ulrich, H. D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 (2003).
Sale, J. E., Lehmann, A. R. & Woodgate, R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nature Rev. Mol. Cell Biol. 13, 141–152 (2012).
Friedberg, E. C. Suffering in silence: the tolerance of DNA damage. Nature Rev. Mol. Cell Biol. 6, 943–953 (2005).
Wang, S. C. et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nature Cell Biol. 8, 1359–1368 (2006).
Yu, Y. et al. Proliferating cell nuclear antigen is protected from degradation by forming a complex with MutT homolog2. J. Biol. Chem. 284, 19310–19320 (2009).
Loeb, L. A. & Monnat, R. J. Jr. DNA polymerases and human disease. Nature Rev. Genet. 9, 594–604 (2008).
Karras, G. I. & Jentsch, S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 141, 255–267 (2010).
Daigaku, Y., Davies, A. A. & Ulrich, H. D. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature 465, 951–955 (2010). References 36 and 37 provided the demonstration that the RAD6 -dependent pathway of DNA damage tolerance can operate outside of S phase.
Jansen, J. G. et al. Mammalian polymerase zeta is essential for post-replication repair of UV-induced DNA lesions. DNA Repair (Amst.) 8, 1444–1451 (2009).
Jansen, J. G. et al. Separate domains of Rev1 mediate two modes of DNA damage bypass in mammalian cells. Mol. Cell. Biol. 29, 3113–3123 (2009).
Edmunds, C. E., Simpson, L. J. & Sale, J. E. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell 30, 519–529 (2008).
Arakawa, H. et al. A role for PCNA ubiquitination in immunoglobulin hypermutation. PLoS Biol. 4, e366 (2006).
Frampton, J. et al. Postreplication repair and PCNA modification in Schizosaccharomyces pombe. Mol. Biol. Cell 17, 2976–2985 (2006).
Leach, C. A. & Michael, W. M. Ubiquitin/SUMO modification of PCNA promotes replication fork progression in Xenopus laevis egg extracts. J. Cell Biol. 171, 947–954 (2005).
Watanabe, K. et al. Rad18 guides Pol η to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 23, 3886–3896 (2004).
Kannouche, P. L., Wing, J. & Lehmann, A. R. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14, 491–500 (2004).
Chang, D. J., Lupardus, P. J. & Cimprich, K. A. Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and mini-chromosome maintenance helicase activities. J. Biol. Chem. 281, 32081–32088 (2006).
Tsuji, Y. et al. Recognition of forked and single-stranded DNA structures by human RAD18 complexed with RAD6B protein triggers its recruitment to stalled replication forks. Genes Cells 13, 343–354 (2008).
Davies, A. A., Huttner, D., Daigaku, Y., Chen, S. & Ulrich, H. D. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein A. Mol. Cell 29, 625–636 (2008).
Hibbert, R. G., Huang, A., Boelens, R. & Sixma, T. K. E3 ligase Rad18 promotes monoubiquitination rather than ubiquitin chain formation by E2 enzyme Rad6. Proc. Natl Acad. Sci. USA 108, 5590–5595 (2011).
Zhang, S. et al. PCNA is ubiquitinated by RNF8. Cell Cycle 7, 3399–3404 (2008).
Lin, J. R., Zeman, M. K., Chen, J. Y., Yee, M. C. & Cimprich, K. A. SHPRH and HLTF act in a damage-specific manner to coordinate different forms of postreplication repair and prevent mutagenesis. Mol. Cell 42, 237–249 (2011).
Terai, K., Abbas, T., Jazaeri, A. A. & Dutta, A. CRL4Cdt2 E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol. Cell 37, 143–149 (2010).
Das-Bradoo, S. et al. Defects in DNA ligase I trigger PCNA ubiquitylation at Lys 107. Nature Cell Biol. 12, 74–79 (2010).
Wagner, S. A. et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics 10, M111.013284 (2011).
Kim, W. et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 (2011).
Hishiki, A. et al. Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen. J. Biol. Chem. 284, 10552–10560 (2009).
Bienko, M. et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 (2005). The identification of ubiquitin-binding domains in Y-family TLS polymerases that facilitate their recruitment to monoubiquitylated PCNA at blocked replication forks.
Plosky, B. S. et al. Controlling the subcellular localization of DNA polymerases ι and η via interactions with ubiquitin. EMBO J. 25, 2847–2855 (2006).
Guo, C. et al. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 26, 8892–8900 (2006).
Guo, C., Tang, T. S., Bienko, M., Dikic, I. & Friedberg, E. C. Requirements for the interaction of mouse Polκ with ubiquitin and its biological significance. J. Biol. Chem. 283, 4658–4664 (2008).
Kannouche, P. et al. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 15, 158–172 (2001).
Sabbioneda, S. et al. Effect of proliferating cell nuclear antigen ubiquitination and chromatin structure on the dynamic properties of the Y-family DNA polymerases. Mol. Biol. Cell 19, 5193–5202 (2008).
Prakash, S., Johnson, R. E. & Prakash, L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74, 317–353 (2005).
Ohashi, E. et al. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9, 523–531 (2004).
Murakumo, Y. et al. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 276, 35644–35651 (2001).
Hibbert, R. G. & Sixma, T. K. Intrinsic flexibility of ubiquitin on proliferating cell nuclear antigen (PCNA) in translesion synthesis. J. Biol. Chem. 287, 39216–39223 (2012).
Freudenthal, B. D., Gakhar, L., Ramaswamy, S. & Washington, M. T. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nature Struct. Mol. Biol. 17, 479–484 (2010).
Hendel, A. et al. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 7, e1002262 (2011).
Szuts, D., Marcus, A. P., Himoto, M., Iwai, S. & Sale, J. E. REV1 restrains DNA polymerase ζ to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo. Nucleic Acids Res. 36, 6767–6780 (2008).
Yang, K., Moldovan, G. L. & D'Andrea, A. D. RAD18-dependent recruitment of SNM1A to DNA repair complexes by a ubiquitin-binding zinc finger. J. Biol. Chem. 285, 19085–19091 (2010).
Saugar, I., Parker, J. L., Zhao, S. & Ulrich, H. D. The genome maintenance factor Mgs1 is targeted to sites of replication stress by ubiquitylated PCNA. Nucleic Acids Res. 40, 245–257 (2012).
Crosetto, N. et al. Human Wrnip1 is localized in replication factories in a ubiquitin-binding zinc finger-dependent manner. J. Biol. Chem. 283, 35173–35185 (2008).
Ulrich, H. D. & Walden, H. Ubiquitin signalling in DNA replication and repair. Nature Rev. Mol. Cell Biol. 11, 479–489 (2010).
Motegi, A. et al. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J. Cell Biol. 175, 703–708 (2006).
Motegi, A. et al. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl Acad. Sci. USA 105, 12411–12416 (2008).
Unk, I. et al. Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination. Proc. Natl Acad. Sci. USA 105, 3768–3773 (2008).
Unk, I. et al. Human SHPRH is a ubiquitin ligase for Mms2–Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc. Natl Acad. Sci. USA 103, 18107–18112 (2006).
Krijger, P. H. et al. HLTF and SHPRH are not essential for PCNA polyubiquitination, survival and somatic hypermutation: existence of an alternative E3 ligase. DNA Repair (Amst.) 10, 438–444 (2011).
Yuan, J., Ghosal, G. & Chen, J. The HARP-like domain-containing protein AH2/ZRANB3 binds to PCNA and participates in cellular response to replication stress. Mol. Cell 47, 410–421 (2012).
Weston, R., Peeters, H. & Ahel, D. ZRANB3 is a structure-specific ATP-dependent endonuclease involved in replication stress response. Genes Dev. 26, 1558–1572 (2012).
Driscoll, R. & Cimprich, K. A. HARPing on about the DNA damage response during replication. Genes Dev. 23, 2359–2365 (2009).
Niimi, A. et al. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl Acad. Sci. USA 105, 16125–16130 (2008).
Gohler, T., Munoz, I. M., Rouse, J. & Blow, J. J. PTIP/Swift is required for efficient PCNA ubiquitination in response to DNA damage. DNA Repair (Amst.) 7, 775–787 (2008).
Yang, X. H., Shiotani, B., Classon, M. & Zou, L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 22, 1147–1152 (2008).
Huang, T. T. et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nature Cell Biol. 8, 339–347 (2006). The discovery that the DUB USP1 counteracts UV light-induced PCNA monoubiquitylation in human cells.
Gallego-Sanchez, A., Andres, S., Conde, F., San-Segundo, P. A. & Bueno, A. Reversal of PCNA ubiquitylation by Ubp10 in Saccharomyces cerevisiae. PLoS Genet. 8, e1002826 (2012).
Faesen, A. C. et al. The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem. Biol. 18, 1550–1561 (2011).
Cohn, M. A. et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell 28, 786–797 (2007).
Lee, K. Y. et al. Human ELG1 regulates the level of ubiquitinated proliferating cell nuclear antigen (PCNA) through its interactions with PCNA and USP1. J. Biol. Chem. 285, 10362–10369 (2010).
Oestergaard, V. H. et al. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol. Cell 28, 798–809 (2007).
Parker, J. L. et al. SUMO modification of PCNA is controlled by DNA. EMBO J. 27, 2422–2431 (2008).
Pfander, B., Moldovan, G. L., Sacher, M., Hoege, C. & Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436, 428–433 (2005). Describes the role of PCNA SUMOylation in recruiting Srs2 during S phase to inhibit recombination.
Krejci, L. et al. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423, 305–309 (2003).
Veaute, X. et al. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423, 309–312 (2003).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005).
Freudenthal, B. D., Brogie, J. E., Gakhar, L., Kondratick, C. M. & Washington, M. T. Crystal structure of SUMO-modified proliferating cell nuclear antigen. J. Mol. Biol. 406, 9–17 (2011).
Armstrong, A. A., Mohideen, F. & Lima, C. D. Recognition of SUMO-modified PCNA requires tandem receptor motifs in Srs2. Nature 483, 59–63 (2012). References 66, 67 and 97 provide structural studies of PCNA modified by ubiquitin and SUMO and reveal that their orientation is on the back face of PCNA.
Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P. & Dikic, I. Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281, 16117–16127 (2006).
Gali, H. et al. Role of SUMO modification of human PCNA at stalled replication fork. Nucleic Acids Res. 40, 6049–6059 (2012).
Moldovan, G. L. et al. Inhibition of homologous recombination by the PCNA-interacting protein PARI. Mol. Cell 45, 75–86 (2012).
Parnas, O. et al. Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA. EMBO J. 29, 2611–2622 (2010).
Yang, K. et al. Regulation of the Fanconi anemia pathway by a SUMO-like delivery network. Genes Dev. 25, 1847–1858 (2011).
Bienko, M. et al. Regulation of translesion synthesis DNA polymerase eta by monoubiquitination. Mol. Cell 37, 396–407 (2010).
Parker, J. L., Bielen, A. B., Dikic, I. & Ulrich, H. D. Contributions of ubiquitin- and PCNA-binding domains to the activity of Polymerase η in Saccharomyces cerevisiae. Nucleic Acids Res. 35, 881–889 (2007).
Woelk, T. et al. Molecular mechanisms of coupled monoubiquitination. Nature Cell Biol. 8, 1246–1254 (2006).
Gohler, T., Sabbioneda, S., Green, C. M. & Lehmann, A. R. ATR-mediated phosphorylation of DNA polymerase η is needed for efficient recovery from UV damage. J. Cell Biol. 192, 219–227 (2011).
Henneke, G., Koundrioukoff, S. & Hubscher, U. Phosphorylation of human Fen1 by cyclin-dependent kinase modulates its role in replication fork regulation. Oncogene 22, 4301–4313 (2003).
Scott, M. T., Morrice, N. & Ball, K. L. Reversible phosphorylation at the C-terminal regulatory domain of p21Waf1/Cip1 modulates proliferating cell nuclear antigen binding. J. Biol. Chem. 275, 11529–11537 (2000).
Havens, C. G. & Walter, J. C. Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25, 1568–1582 (2011).
Arias, E. E. & Walter, J. C. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nature Cell Biol. 8, 84–90 (2006).
Higa, L. A. et al. L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle 5, 1675–1680 (2006).
Hu, J. & Xiong, Y. An evolutionarily conserved function of proliferating cell nuclear antigen for Cdt1 degradation by the Cul4–Ddb1 ubiquitin ligase in response to DNA damage. J. Biol. Chem. 281, 3753–3756 (2006).
Nishitani, H. et al. Two E3 ubiquitin ligases, SCF–Skp2 and DDB1–Cul4, target human Cdt1 for proteolysis. EMBO J. 25, 1126–1136 (2006).
Senga, T. et al. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J. Biol. Chem. 281, 6246–6252 (2006). References 110–114 report the discovery that PCNA is required as a molecular platform for the ubiquitylation and subsequent degradation of a PIP box-dependent interactor, CDT1.
Oda, H. et al. Regulation of the histone H4 monomethylase PR-Set7 by CRL4Cdt2-mediated PCNA-dependent degradation during DNA damage. Mol. Cell 40, 364–376 (2010).
Jorgensen, S. et al. SET8 is degraded via PCNA-coupled CRL4CDT2 ubiquitylation in S phase and after UV irradiation. J. Cell Biol. 192, 43–54 (2011).
Centore, R. C. et al. CRL4Cdt2-mediated destruction of the histone methyltransferase Set8 prevents premature chromatin compaction in S phase. Mol. Cell 40, 22–33 (2010).
Abbas, T. et al. CRL4Cdt2 regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol. Cell 40, 9–21 (2010).
Raman, M., Havens, C. G., Walter, J. C. & Harper, J. W. A genome-wide screen identifies p97 as an essential regulator of DNA damage-dependent CDT1 destruction. Mol. Cell 44, 72–84 (2011).
Havens, C. G. & Walter, J. C. Docking of a specialized PIP box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol. Cell 35, 93–104 (2009).
Michishita, M. et al. Positively charged residues located downstream of PIP box, together with TD amino acids within PIP box, are important for CRL4Cdt2-mediated proteolysis. Genes Cells 16, 12–22 (2011).
Havens, C. G. et al. Direct role for proliferating cell nuclear antigen in substrate recognition by the E3 ubiquitin ligase CRL4Cdt2. J. Biol. Chem. 287, 11410–11421 (2012).
Shibutani, S. T. et al. Intrinsic negative cell cycle regulation provided by PIP box- and Cul4Cdt2-mediated destruction of E2f1 during S phase. Dev. Cell 15, 890–900 (2008).
Kim, S. H. & Michael, W. M. Regulated proteolysis of DNA polymerase eta during the DNA-damage response in C. elegans. Mol. Cell 32, 757–766 (2008).
Jung, Y. S., Liu, G. & Chen, X. Pirh2 E3 ubiquitin ligase targets DNA polymerase η for 20S proteasomal degradation. Mol. Cell. Biol. 30, 1041–1048 (2010).
Povlsen, L. K. et al. Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nature Cell Biol. 14, 1089–1098 (2012). The first systems-wide analysis of proteins regulated by ubiquitylation in response to DNA damage in human cells.
Soria, G., Speroni, J., Podhajcer, O. L., Prives, C. & Gottifredi, V. p21 differentially regulates DNA replication and DNA-repair-associated processes after UV irradiation. J. Cell Sci. 121, 3271–3282 (2008).
Acs, K. et al. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nature Struct. Mol. Biol. 18, 1345–1350 (2011).
Meerang, M. et al. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nature Cell Biol. 13, 1376–1382 (2011).
Mosbech, A. et al. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nature Struct. Mol. Biol. 19, 1084–1092 (2012).
Davis, E. J. et al. DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nature Struct. Mol. Biol. 19, 1093–1100 (2012).
Ghosal, G., Leung, J. W., Nair, B. C., Fong, K. W. & Chen, J. Proliferating cell nuclear antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis. J. Biol. Chem. 287, 34225–34233 (2012).
Centore, R. C., Yazinski, S. A., Tse, A. & Zou, L. Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol. Cell 46, 625–635 (2012).
Juhasz, S. et al. Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance. Nucleic Acids Res. 40, 10795–10808 (2012).
Zeman, M. K. & Cimprich, K. A. Finally, polyubiquitinated PCNA gets recognized. Mol. Cell 47, 333–334 (2012).
Xie, K., Doles, J., Hemann, M. T. & Walker, G. C. Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl Acad. Sci. USA 107, 20792–20797 (2010).
Doles, J. et al. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc. Natl Acad. Sci. USA 107, 20786–20791 (2010). References 136 and 137 show that TLS has a major role in mediating acquired chemoresistance and that inhibition of TLS may provide a therapeutic target for tumours that are resistant to chemotherapy.
Punchihewa, C. et al. Identification of small molecule proliferating cell nuclear antigen (PCNA) inhibitor that disrupts interactions with PIP-box proteins and inhibits DNA replication. J. Biol. Chem. 287, 14289–14300 (2012).
Ulrich, H. D. Timing and spacing of ubiquitin-dependent DNA damage bypass. FEBS Lett. 585, 2861–2867 (2011).
Masutani, C. et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399, 700–704 (1999).
Johnson, R. E., Kondratick, C. M., Prakash, S. & Prakash, L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285, 263–265 (1999). References 140 and 141 demonstrate that xeroderma pigmentosum variant is caused by mutations in the gene encoding Pol η.
Guo, C. et al. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23, 265–271 (2006).
Ulrich, H. D. & Jentsch, S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. EMBO J. 19, 3388–3397 (2000).
Hofmann, R. M. & Pickart, C. M. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653 (1999).
Acknowledgements
The authors apologize to those whose important findings could not be cited owing to page limitations. They thank J. Lukas for critical reading of the manuscript. Work in the authors' laboratory is funded by grants from the Novo Nordisk Foundation, the Danish Medical Research Council, the Lundbeck Foundation and the Danish Cancer Society.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- AAA+ ATPase
-
Superfamily of proteins that use ATP hydrolysis to remodel or translocate macromolecules in diverse cellular processes such as DNA replication, protein degradation, membrane fusion and signal transduction.
- Isothermal titration calorimetry
-
(ITC). Gold standard biophysical technique to quantitatively determine the thermodynamic parameters of molecular interactions in solution.
- Writers
-
Enzymes that can add chemical modifications to DNA or proteins.
- Readers
-
Factors that bind proteins or DNA only when certain chemical modifications are present (or absent). Readers often recognize similar modifications through particular protein domains.
- RAD6 epistasis group
-
Genes required for post-replication repair in Saccharomyces cerevisiae, including RAD6, RAD18, RAD5, methyl methanesulphonate sensitive 2 (MMS2) and ubiquitin-conjugating enzyme 13 (UBC13) (all of which are enzymes involved in protein ubiquitylation), as well as proliferating cell nuclear antigen (PCNA) and translesion DNA synthesis polymerases.
- E2 ubiquitin-conjugating enzyme
-
A family of enzymes (comprising ∼40 members in mammalian cells) that, in conjunction with an E3 ubiquitin ligase, mediates the transfer of activated ubiquitin from the E2 active site Cys to a Lys residue in a target protein.
- Cisplatin
-
A platinum-containing chemotherapeutic drug (cis-diamminedichloropltinum(II)) that induces DNA crosslinks (primarily intrastrand crosslinks and to a lesser extent interstrand crosslinks).
- Erasers
-
Enzymes that can removechemical modifications from DNA or proteins.
- UvrD-like domain
-
Domain found in a wide range of proteins that belong to the UvrD-like DNA helicase family, which are ATPases that unwind DNA with a 3′-5′ polarity.
Rights and permissions
About this article
Cite this article
Mailand, N., Gibbs-Seymour, I. & Bekker-Jensen, S. Regulation of PCNA–protein interactions for genome stability. Nat Rev Mol Cell Biol 14, 269–282 (2013). https://doi.org/10.1038/nrm3562
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3562
This article is cited by
-
BLVRA exerts its biological effects to induce malignant properties of hepatocellular carcinoma cells via Wnt/β-catenin pathway
Journal of Molecular Histology (2024)
-
ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study
Oncogene (2023)
-
Dietary alpha-lipoic acid boosts growth, immune-antioxidant traits, behavior, and transcriptomes of antioxidant, apoptosis, and immune-related genes to combat cold stress in Nile tilapia (Oreochromis niloticus)
Aquaculture International (2023)
-
PCLAF promotes neuroblastoma G1/S cell cycle progression via the E2F1/PTTG1 axis
Cell Death & Disease (2022)
-
Influence of the treatment with the antineoplastic agents 5-fluorouracil and cisplatin on the severity of experimental periodontitis in rats
Supportive Care in Cancer (2022)