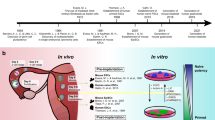
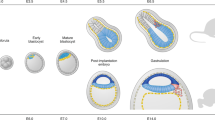
Abstract
The cell biology of the early processes of mammalian embryogenesis, such as germ-layer formation, has been technically challenging to study owing to the size and accessibility of mammalian embryos. Embryonic stem cells, which can generate the three germ layers in vitro, are useful for studying embryogenesis at the cellular level. So, how can the study of embryonic stem cells and their differentiation provide a deeper understanding of the cell biology of early development?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dickinson, M. Multimodal imaging of mouse development: tools for the postgenomic era. Dev. Dyn. 235, 2386–2400 (2006).
Jones, E. A. et al. Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis 34, 228–235 (2002).
Evans, M. & Kaufman, M. Establishment in culture of pluripotent cells from mouse embryos. Nature 292, 154–156 (1981).
Martin, G. Isolation of pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 (1981).
Thomson, J. A. et al. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 55, 254–259 (1996).
Boyer, L. A., Mathur, D. & Jaenisch, R. Molecular control of pluripotency. Curr. Opin. Genet. Dev. 16, 455–462 (2006).
Niwa, H. How is pluripotency determined and maintained? Development 134, 635–646 (2007).
Nichols, J. et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 (1998).
Mitsui, K. et al. The homeoprotein NANOG is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 (2003).
Chambers, I. et al. Functional expression cloning of NANOG, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 (2003).
Avilion, A. A. et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 (2003).
Niwa, H. et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123, 917–929 (2005).
Suzuki, A. et al. NANOG binds to SMAD1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 10294–10299 (2006).
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003).
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Loh, Y. H. et al. The OCT4 and NANOG transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genet. 38, 431–440 (2006).
Azuara, V. et al. Chromatin signatures of pluripotent cell lines. Nature Cell Biol. 8, 532–538 (2006).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 (2006).
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006).
Lee, T. I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Takahashi, K., Mitsui, K. & Yamanaka, S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature 423, 541–545 (2003).
Takahashi, K., Murakami, M. & Yamanaka, S. Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem. Soc. Trans 33, 1522–1525 (2005).
Cartwright, P. et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132, 885–896 (2005).
Tanaka, Y., Patestos, N. P., Maekawa, T. & Ishii, S. B-myb is required for inner cell mass formation at an early stage of development. J. Biol. Chem. 274, 28067–28070 (1999).
Doetschman, T. C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 87, 27–45 (1985).
Nakano, T., Kodama, H. & Honjo, T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265, 1098–1101 (1994).
Nishikawa, S. I., Nishikawa, S., Hirashima, M., Matsuyoshi, N. & Kodama, H. Progressive lineage analysis by cell sorting and culture identifies FLK1+ VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747–1757 (1998).
Fujikura, J. et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16, 784–789 (2002).
Martin, G. R. & Evans, M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc. Natl Acad. Sci. USA 72, 1441–1445 (1975).
Coucouvanis, E. & Martin, G. R. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 83, 279–287 (1995).
Hubner, K. et al. Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256 (2003).
Toyooka, Y., Tsunekawa, N., Akasu, R. & Noce, T. Embryonic stem cells can form germ cells in vitro. Proc. Natl Acad. Sci. USA 100, 11457–11462 (2003).
Geijsen, N. et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427, 148–154 (2004).
Axelrod, H. R. Embryonic stem cell lines derived from blastocysts by a simplified technique. Dev. Biol. 101, 225–228 (1984).
de Pooter, R. F., Cho, S. K., Carlyle, J. R. & Zuniga-Pflucker, J. C. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood 102, 1649–1653 (2003).
Sakurai, H. et al. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells 24, 575–586 (2006).
Kawasaki, H. et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40 (2000).
Kawasaki, H. et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl Acad. Sci. USA 99, 1580–1585 (2002).
Ueno, M. et al. Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc. Natl Acad. Sci. USA 103, 9554–9559 (2006).
Roy, N. S. et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by co-culture with telomerase-immortalized midbrain astrocytes. Nature Med. 12, 1259–1268 (2006).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006).
Ying, Q. L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature Biotech. 21, 183–186 (2003).
Tada, S. et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363–4374 (2005).
Yasunaga, M. et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nature Biotech. 23, 1542–1550 (2005).
Kabrun, N. et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124, 2039–2048 (1997).
Ng, E. S. et al. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development 132, 873–884 (2005).
Chazaud, C., Yamanaka, Y., Pawson, T. & Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 10, 615–624 (2006).
Johansson, B. M. & Wiles, M. V. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol. Cell. Biol. 15, 141–151 (1995).
Lindsley, R. C., Gill, J. G., Kyba, M., Murphy, T. L. & Murphy, K. M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133, 3787–3796 (2006).
Gadue, P., Huber, T. L., Paddison, P. J. & Keller, G. M. Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 16806–16811 (2006).
Aiba, K. et al. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells 24, 889–895 (2006).
Chen, S. et al. Self-renewal of embryonic stem cells by a small molecule. Proc. Natl Acad. Sci. USA 103, 17266–17271 (2006).
Michiels, F. et al. Arrayed adenoviral expression libraries for functional screening. Nature Biotech. 20, 1154–1157 (2002).
Mousses, S. et al. RNAi microarray analysis in cultured mammalian cells. Genome Res. 13, 2341–2347 (2003).
Neumann, B. et al. High-throughput RNAi screening by time-lapse imaging of live human cells. Nature Methods 3, 385–390 (2006).
Kuschel, C. et al. Cell adhesion profiling using extracellular matrix protein microarrays. Biotechniques 40, 523–531 (2006).
Wakayama, S. et al. Equivalency of nuclear transfer-derived embryonic stem cells to those derived from fertilized mouse blastocysts. Stem Cells 24, 2023–2033 (2006).
Acknowledgements
The work of the Laboratory of Stem Cell Research is supported by grants from the Leading Project for Realization of Regenerative Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Embryonic carcinoma
-
A type of testicular cancer that maintains the potential to give rise to mature tissues.
- Mesoderm
-
The middle germ layer of the developing embryo that occupies an intermediate position between the ectoderm and the endoderm. It gives rise to the skeleton, muscles and connective tissue.
- Pluripotent
-
Embryonic stem cells that are able to form all of the cell lineages of the body, including germ cells, and some or even all extra-embryonic cell types.
- Polycomb
-
A class of proteins — originally described in Drosophila melanogaster — that maintains the stable and heritable repression of several genes.
- Primitive endoderm
-
The extra-embryonic tissue that gives rise to the visceral and parietal endoderm; it diverges directly from the inner cell mass to form the outer layer of the embryo.
- Teratoma
-
Tumours that contain various differentiated cells from all three primary germ layers.
- Trophectoderm
-
The outer layer of the blastocyst.
Rights and permissions
About this article
Cite this article
Nishikawa, SI., Jakt, L. & Era, T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat Rev Mol Cell Biol 8, 502–507 (2007). https://doi.org/10.1038/nrm2189
Issue Date:
DOI: https://doi.org/10.1038/nrm2189
This article is cited by
-
Feeder cell–dependent primary culture of single blastula–derived embryonic cell lines from marine medaka (Oryzias dancena)
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering
Cellular and Molecular Life Sciences (2020)
-
Spatiotemporal patterning of EpCAM is important for murine embryonic endo- and mesodermal differentiation
Scientific Reports (2018)
-
HSP60 is required for stemness and proper differentiation of mouse embryonic stem cells
Experimental & Molecular Medicine (2018)
-
AIMP3 depletion causes genome instability and loss of stemness in mouse embryonic stem cells
Cell Death & Disease (2018)