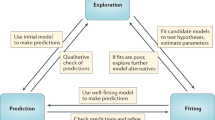
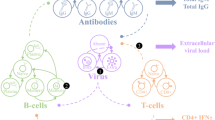
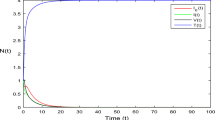
Key Points
-
Modelling the effects of antiretroviral therapy on human immunodeficiency virus (HIV) infection has shown that HIV replicates rapidly and is cleared rapidly in chronically infected individuals. Four distinct timescales have been identified: virion clearance, hours; death of productively infected CD4+ T cells, days; death of long-lived infected cells, weeks; loss of latently infected cells, months to years; and loss of HIV from the FDC network, months to years.
-
Models of virus infection have been applied to the chronic stages of hepatitis C virus (HCV), hepatitis B virus (HBV) and cytomegalovirus (CMV) infection, and show that all three infections are characterized by rapid dynamics.
-
The efficacy and mode of action of antiviral drugs can sometimes be shown from viral-dynamic studies; the relative efficacy of different drug doses and treatment regimes can be estimated from viral-decay curves; and the rapid decay of HCV RNA suggests that interferon blocks HCV production in a dose-dependent manner.
-
Modelling antiviral immune responses is still in its infancy, but it raises interesting quantitative questions about both cytopathic and non-cytopathic effects of CD8 T+ cells in controlling viral infections.
-
The dynamics of lymphocyte populations in HIV-infected and -uninfected individuals have been elucidated through labelling studies combined with models.
-
Future quantitative understanding of the immune system might be speeded by a combination of experiment, formation of immunological databases, modelling and computer simulation.
Abstract
During the past 6 years, there have been substantial advances in our understanding of human immunodeficiency virus 1 and other viruses, such as hepatitis B virus and hepatitis C virus, that cause chronic infection. The use of mathematical modelling to interpret experimental results has made a significant contribution to this field. Mathematical modelling is also improving our understanding of T-cell dynamics and the quantitative events that underlie the immune response to pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Perelson, A. S. & Weisbuch, G. Immunology for physicists. Rev. Mod. Phys. 69, 1219–1267 (1997).Extensive review of modelling in immunology.
Perelson, A. S. & Oster, G. F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self–non-self discrimination. J. Theor. Biol. 81, 645–670 (1979).
Perelson, A. S. in Cell Surface Dynamics: Concepts and Models (eds Perelson, A. S., DeLisi, C. & Weigel, F. W.) 223–276 (Marcel Dekker, New York, 1984).
Kepler, T. B. & Perelson, A. S. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol. Today 14, 412–415 (1993).
De Boer, R. J. & Perelson, A. S. How diverse should the immune system be? Proc. R. Soc. Lond. B 252, 171–175 (1993).
Percus, J. K., Percus, O. E. & Perelson, A. S. Predicting the size of the T-cell receptor and antibody combining region from consideration of efficient self–nonself discrimination. Proc. Natl Acad. Sci. USA 90, 1691–1695 (1993).
McKeithan, T. W. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl Acad. Sci. USA 92, 5042–5046 (1995).
McLean, A. R., Rosado, M. M., Agenes, F., Vasconcellos, R. & Freitas, A. A. Resource competition as a mechanism for B cell homeostasis. Proc. Natl Acad. Sci. USA 94, 5792–5797 (1997).
Borghans, J. A., De Boer, R. J., Sercarz, E. & Kumar, V. T cell vaccination in experimental autoimmune encephalomyelitis: a mathematical model. J. Immunol. 161, 1087–1093 (1998).
Smith, D. J., Forrest, S., Ackley, D. H. & Perelson, A. S. Variable efficacy of repeated annual influenza vaccination. Proc. Natl Acad. Sci. USA 96, 14001–14006 (1999).Shows, through a large-scale simulation model of the humoral immune response, that variable efficacy of influenza vaccine in repeat vaccinees can be explained by a memory response to a prior vaccine or infection; that is, original antigenic sin.
Detours, V. & Perelson, A. S. Explaining high alloreactivity as a quantitative consequence of affinity-driven thymocyte selection. Proc. Natl Acad. Sci. USA 96, 5153–5158 (1999).
Kesmir, C. & De Boer, R. J. A mathematical model on germinal center kinetics and termination. J. Immunol. 163, 2463–2469 (1999).
Borghans, J. A., Taams, L. S., Wauben, M. H. & de Boer, R. J. Competition for antigenic sites during T cell proliferation: a mathematical interpretation of in vitro data. Proc. Natl Acad. Sci. USA 96, 10782–10787 (1999).
Borghans, J. A., Noest, A. J. & De Boer, R. J. How specific should immunological memory be? J. Immunol. 163, 569–575 (1999).
Segel, L. A. & Bar-Or, R. L. On the role of feedback in promoting conflicting goals of the adaptive immune system. J. Immunol. 163, 1342–1349 (1999).
Detours, V. & Perelson, A. S. The paradox of alloreactivity and self MHC restriction: quantitative analysis and statistics. Proc. Natl Acad. Sci. USA 97, 8479–8483 (2000).
Hlavacek, W. S., Redondo, A., Metzger, H., Wofsy, C. & Goldstein, B. Kinetic proofreading models for cell signaling predict ways to escape kinetic proofreading. Proc. Natl Acad. Sci. USA 98, 7295–7300 (2001).
Ho, D. D. et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126 (1995).
Wei, X. et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373, 117–122 (1995).References 18 and 19 are the key papers showing that HIV replicates and is cleared rapidly during chronic HIV-1 infection.
Perelson, A. S., Neumann, A. U., Markowitz, M., Leonard, J. M. & Ho, D. D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271, 1582–1586 (1996).This paper provided estimates of the lifespan of productively infected CD4+ lymphocytes and the rate of clearance of free virions.
Coffin, J. M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267, 483–489 (1995).
Wain-Hobson, S. AIDS. Virological mayhem. Nature 373, 102 (1995).
Perelson, A. S., Essunger, P. & Ho, D. D. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS 11, S17–S24 (1997).
Nowak, M. A. & May, R. M. Virus Dynamics: Mathematical Principles of Immunology and Virology (Oxford Univ. Press, 2000).Monograph that reviews the field of viral dynamic modelling.
Ho, D. D. Viral counts count in HIV infection. Science 272, 1124–1125 (1996).
Perelson, A. S. & Nelson, P. W. Mathematical analysis of HIV-1 dynamics in vivo. SIAM Rev. 41, 3–44 (1999).A review of the mathematics used in modelling HIV infection.
Wu, H., Ruan, P., Ding, A. A., Sullivan, J. L. & Luzuriaga, K. Inappropriate model-fitting methods may lead to significant underestimates of viral decay rates in HIV dynamic studies. J. Acquir. Immune Defic. Syndr. 21, 426–428 (1999).
Ramratnam, B. et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 354, 1782–1785 (1999).
Mansky, L. M. & Temin, H. M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 69, 5087–5094 (1995).
Bonhoeffer, S., May, R. M., Shaw, G. M. & Nowak, M. A. Virus dynamics and drug therapy. Proc. Natl Acad. Sci. USA 94, 6971–6976 (1997).
Wein, L. M., D'Amato, R. M. & Perelson, A. S. Mathematical analysis of antiretroviral therapy aimed at HIV-1 eradication or maintenance of low viral loads. J. Theor. Biol. 192, 81–98 (1998).
Ding, A. A. & Wu, H. Relationships between antiviral treatment effects and biphasic viral decay rates in modeling HIV dynamics. Math. Biosci. 160, 63–82 (1999).
Mueller, B. U. et al. Individual prognoses of long-term responses to antiretroviral treatment based on virological, immunological and pharmacological parameters measured during the first week under therapy. AIDS 12, F191–F196 (1998).
Mittler, J. et al. Short-term measures of relative efficacy predict longer-term reductions in human immunodeficiency virus type 1 RNA levels following nelfinavir monotherapy. Antimicrob. Agents Chemother. 45, 1438–1443 (2001).
Louie, M. et al. Using viral dynamics to document the greater antiviral potency of a regime containing lopinavir/ritonavir, efavirenz, tenofovir, and lamivudine relative to standard therapy. 8th Conf. Retroviruses Opportunistic Infect. Abstr. 383 (2001).
Perelson, A. S. et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387, 188–191 (1997).This paper reported that the decline in plasma HIV-1 RNA after the initiation of therapy has two phases. The second phase was attributed to long-lived infected cells, release of virus from tissue reservoirs, or the activation of latently infected cells. Indicates that the cells responsible for the second phase could be eradicated after 2–3 years of 100% effective therapy.
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Chun, T. W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997).
Zhang, L. et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340, 1605–1613 (1999).
Finzi, D. et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature Med. 5, 512–517 (1999).
Wong, J. K. et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997).
Chun, T. W. et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 94, 13193–13197 (1997).
Furtado, M. R. et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340, 1614–1622 (1999).
Dornadula, G. et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282, 1627–1632 (1999).
Sharkey, M. E. et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nature Med. 6, 76–81 (2000).
Siliciano, J. D. & Siliciano, R. F. Latency and viral persistence in HIV-1 infection. J. Clin. Invest. 106, 823–825 (2000).
Hlavacek, W. S., Wofsy, C. & Perelson, A. S. Dissociation of HIV-1 from follicular dendritic cells during HAART: mathematical analysis. Proc. Natl Acad. Sci. USA 96, 14681–14686 (1999).Shows that complete elimination of HIV-1 from follicular dendritic cells can take as long as a decade if virions do not degrade.
Hlavacek, W. S., Stilianakis, N. I., Notermans, D. W., Danner, S. A. & Perelson, A. S. Influence of follicular dendritic cells on decay of HIV during antiretroviral therapy. Proc. Natl Acad. Sci. USA 97, 10966–10971 (2000).
Hlavacek, W. S., Stilianakis, N. I. & Perelson, A. S. Influence of follicular dendritic cells on HIV dynamics. Philos Trans R Soc Lond B Biol Sci 355, 1051–1058 (2000).
Smith, B. A. et al. Persistence of infectious HIV on follicular dendritic cells. J. Immunol. 166, 690–696 (2001).
Neumann, A. U. et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282, 103–107 (1998).Shows that HCV, like HIV, has rapid production and clearance. Also indicates that interferon causes rapid HCV clearance mainly by blocking production of virions from infected cells in a dose-dependent manner.
Bekkering, F. C. et al. Ultrarapid hepatitis C virus clearance by daily high-dose interferon in non-responders to standard therapy. J. Hepatol. 28, 960–964 (1998).
Yasui, K. et al. Dynamics of hepatitis C viremia following interferon-α administration. J. Infect. Dis. 177, 1475–1479 (1998).
Neumann, A. U. et al. Differences in viral dynamics between genotypes 1 and 2 of hepatitis C virus. J. Infect. Dis. 182, 28–35 (2000).
Nowak, M. A. et al. Viral dynamics in hepatitis B virus infection. Proc. Natl Acad. Sci. USA 93, 4398–4402 (1996).First paper to analyse the dynamics of HBV infection.
Tsiang, M., Rooney, J. F., Toole, J. J. & Gibbs, C. S. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29, 1863–1869 (1999).
Lau, G. K. et al. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology 32, 394–399 (2000).
Lewin, S. R. et al. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology 34, 1012–1020 (2001).
Guidotti, L. G. et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 284, 825–829 (1999).
Whalley, S. A. et al. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193, 847–854 (2001).
Emery, V. C., Cope, A. V., Bowen, E. F., Gor, D. & Griffiths, P. D. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 190, 177–182 (1999).
Emery, V. C. & Griffiths, P. D. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc. Natl Acad. Sci. USA 97, 8039–8044 (2000).
Phillips, A. N. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science 271, 497–499 (1996).
Nowak, M. A. & Bangham, C. R. Population dynamics of immune responses to persistent viruses. Science 272, 74–79 (1996).
Koup, R. A. et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655 (1994).
Stafford, M. A. et al. Modeling plasma virus concentration during primary HIV infection. J. Theor. Biol. 203, 285–301 (2000).
Welsh, R. M. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193, F19–F22 (2001).
Xiong, Y. et al. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75, 3028–3033 (2001).
Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Walsh, C. M. et al. Immune function in mice lacking the perforin gene. Proc. Natl Acad. Sci. USA 91, 10854–10858 (1994).
DeBoer, R. J. et al. Recruitment times, proliferation, and apoptosis rates during the CD8+ T cell response to LCMV. J. Virol. 75, 10663–10669 (2001).
Bocharov, G. A. Modelling the dynamics of LCMV infection in mice: conventional and exhaustive CTL responses. J. Theor. Biol. 192, 283–308 (1998).
Dahari, H., Major, M., Mihalik, K., Feinstone, S. & Neumann, A. U. Lack of virus cytopathicity but strong IFN and cellular immune responses during primary HCV infections in chimpanzees. Hepatology 32, 302A (2000).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Wodarz, D. et al. A new theory of cytotoxic T-lymphocyte memory: implications for HIV treatment. Philos Trans R Soc Lond B Biol Sci 355, 329–343 (2000).
Wodarz, D. & Nowak, M. A. Correlates of cytotoxic T-lymphocyte-mediated virus control: implications for immunosuppressive infections and their treatment. Philos Trans R Soc Lond B Biol Sci 355, 1059–1070 (2000).
Wodarz, D. & Jansen, V. A. The role of T cell help for anti-viral CTL responses. J. Theor. Biol. 211, 419–432 (2001).
Mellors, J. W. et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272, 1167–1170 (1996).
Muller, V., Maree, A. F. M. & De Boer, R. J. Small variations in multiple parameters account for wide variations in HIV-1 set points: a novel modelling approach. Proc R Soc Lond B Biol Sci 268, 235–242 (2001).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Jin, X. et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 (1999).
Cocchi, F. et al. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV- suppressive factors produced by CD8+ T cells. Science 270, 1811–1815 (1995).
Mackewicz, C. E., Blackbourn, D. J. & Levy, J. A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl Acad. Sci. USA 92, 2308–2312 (1995).
Herz, A. V., Bonhoeffer, S., Anderson, R. M., May, R. M. & Nowak, M. A. Viral dynamics in vivo: limitations on estimates of intracellular delay and virus decay. Proc. Natl Acad. Sci. USA 93, 7247–7251 (1996).
Mittler, J. E., Sulzer, B., Neumann, A. U. & Perelson, A. S. Influence of delayed viral production on viral dynamics in HIV-1 infected patients. Math. Biosci. 152, 143–163 (1998).
Mittler, J. E., Markowitz, M., Ho, D. D. & Perelson, A. S. Improved estimates for HIV-1 clearance rate and intracellular delay. AIDS 13, 1415–1417 (1999).
Nelson, P. W., Murray, J. D. & Perelson, A. S. A model of HIV-1 pathogenesis that includes an intracellular delay. Math. Biosci. 163, 201–215 (2000).
Nelson, P. W., Mittler, J. E. & Perelson, A. S. Effect of drug efficacy and the eclipse phase of the viral life cycle on estimates of HIV viral dynamic parameters. J. Acquir. Immune Defic. Syndr. 26, 405–412 (2001).
Levy, J. A. HIV and the Pathogenesis of AIDS (American Society for Microbiology, Washington DC, 1998).
Macallan, D. C. et al. Measurement of cell proliferation by labeling of DNA with stable isotope-labeled glucose: studies in vitro, in animals, and in humans. Proc. Natl Acad. Sci. USA 95, 708–713 (1998).
Hellerstein, M. K. Measurement of T-cell kinetics: recent methodologic advances. Immunol. Today 20, 438–441 (1999).
Mohri, H., Bonhoeffer, S., Monard, S., Perelson, A. S. & Ho, D. D. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279, 1223–1227 (1998).
Mohri, H. et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194, 1277–1287 (2001).T-cell kinetics in uninfected and HIV-infected humans determined by stable isotope labelling and modelling of the labelling kinetics.
McCune, J. M. et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Invest. 105, R1–R8 (2000).
Bonhoeffer, S., Mohri, H., Ho, D. & Perelson, A. S. Quantification of cell turnover kinetics using 5-bromo-2′-deoxyuridine. J. Immunol. 164, 5049–5054 (2000).
Hellerstein, M. et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nature Med. 5, 83–89 (1999).
Rouzine, I. M. & Coffin, J. M. T cell turnover in SIV infection. Science 284, 555b (1999). | Article |
Grossman, Z., Herberman, R. B. & Dimitrov, D. S. T cell turnover in SIV infection. Science 284, 555a (1999).
Celada, F. & Seiden, P. E. A computer model of cellular interactions in the immune system. Immunol. Today 13, 56–62 (1992).
Smith, D. J., Forrest, S., Ackley, D. H. & Perelson, A. S. Using lazy evaluation to simulate realistic-size repertoires in models of the immune system. Bull. Math. Biol. 60, 647–658 (1998).
Ribiero, R., Mohri, H., Ho, D. D. & Perelson, A. S. Modeling deuterated glucose labeling of T lymphocytes. Bull. Math. Biol. (in the press).
Acknowledgements
This work was supported by the US Department of Energy and the National Institutes of Health. I thank R. Ribeiro for helpful comments.
Author information
Authors and Affiliations
Related links
Glossary
- CD4 COUNT
-
A normal CD4 count is 1,000 per μl, with a range of 600–1,400 per μl. The count falls during primary infection, then returns to near or lower than normal levels. It then slowly falls, taking many years to reach the level of 200 per μl that characterizes AIDS.
- V
-
Various notations have been used in modelling viral infections. V, the concentration of free virus, in the case of HIV, is measured in units of HIV-1 RNA per ml. As there are two RNAs per virion, the true concentration of virions is half the RNA concentration.
- I
-
Productively infected cells have here been denoted I. In the HIV literature, as there are different types of infected cells, T*, has been used instead of I to denote the population of productively infected CD4+ T cells. In the case of HCV and HBV, I denotes infected hepatocytes.
- NONLINEAR LEAST SQUARES
-
A procedure that estimates the parameters in a model by minimizing the differences between model predictions and data.
- FOLLICULAR DENDRITIC CELLS
-
(FDCs). Specialized non-haematopoietic stromal cells that reside in lymphoid follicles and germinal centres. These cells have long dendrites and carry intact antigen on their surface for long periods of time.
- INTERFERON-α
-
(IFN-α). A cytokine secreted by many cell types in an early response to viral infection. IFN-α has pleiotropic antiviral effects, including inhibition of protein synthesis and DNA replication, enhanced antigen presentation and natural killer-cell activation.
- HCV GENOTYPE
-
The nucleotide sequence of HCV is highly variable, with the most divergent isolates sharing only 60% nucleotide sequence homology. On the basis of sequence similarity, isolates have been grouped into six main types, called genotypes.
- MHC TETRAMERS
-
Reagents composed of four MHC–peptide complexes linked by biotinylation, which can be fluorescently labelled and used to track antigen-specific T cells by flow cytometry.
Rights and permissions
About this article
Cite this article
Perelson, A. Modelling viral and immune system dynamics. Nat Rev Immunol 2, 28–36 (2002). https://doi.org/10.1038/nri700
Issue Date:
DOI: https://doi.org/10.1038/nri700
This article is cited by
-
A novel simulation-based analysis of a stochastic HIV model with the time delay using high order spectral collocation technique
Scientific Reports (2024)
-
Mathematical modelling of HIV-1 transcription inhibition: a comparative study between optimal control and impulsive approach
Computational and Applied Mathematics (2023)
-
On a three-dimensional and two four-dimensional oncolytic viro-therapy models
Boletín de la Sociedad Matemática Mexicana (2023)
-
A Continuation Technique for Maximum Likelihood Estimators in Biological Models
Bulletin of Mathematical Biology (2023)
-
Could Mathematics be the Key to Unlocking the Mysteries of Multiple Sclerosis?
Bulletin of Mathematical Biology (2023)