Key Points
-
By providing a global and integrated view of the host response to infection, functional genomic and systems-biology approaches are contributing to our understanding of RNA virus–host interactions. One area in which these approaches are being put to particularly good use is in shedding new light on the components of innate antiviral defence mechanisms and the viral strategies used to regulate or overcome them.
-
Genomic analyses have helped to reveal virus-specific differences in the way that viral recognition through pathogen-recognition receptors (PRRs) initiates intracellular signalling cascades. Whereas influenza virus appears to signal primarily through retinoic-acid-inducible gene I (RIG-I), West Nile virus signals through both RIG-I and melanoma differentiation-associated gene 5 (MDA5). Both viruses induce the expression of interferon (IFN)-regulatory factor 3 (IRF3) target genes and IFN-stimulated genes (ISGs).
-
Genomic analyses have provided a comprehensive view of the transcriptional programmes that are induced by Toll-like receptor (TLR) activation. One transcriptional profile is universally activated by all TLRs and a second profile is specific to TLR3 and TLR4. Nuclear factor-κB (NF-κB) is the key regulator of the universal response, which occurs early after TLR stimulation, and the IFN-stimulated response element (ISRE) is the key component of the TLR3/TLR4 response, which is induced after the NF-κB response.
-
Some highly virulent viruses, such as Ebola virus and rabies virus, are successful at inhibiting ISG expression, resulting in the marked suppression of genes in key innate antiviral pathways, including those mediated by IRF3. There seems to be a correlation between the antagonism of the IFN response and virulence.
-
Genomic analyses of the host response to the reconstructed 1918 pandemic influenza virus have revealed similarities and differences to contemporary influenza virus infection. Contemporary and 1918 influenza viruses each trigger an innate immune response that includes the expression of NF-κB and IRF3 target genes, and both viruses trigger a robust cytokine response that attracts immune-cell infiltration to infected tissues. Unlike contemporary virus strains, in which the early response to infection is resolved, the innate immune response triggered by the 1918 influenza virus is characterized by a strong and sustained induction that is associated with massive tissue damage and death.
-
Global gene-expression profiling has revealed that many effective, attenuated live-virus vaccines transiently induce a stronger type I IFN response than the cognate pathogen, and therefore implicates modulation of this response as an important strategy in rational vaccine design.
Abstract
Although often encoding fewer than a dozen genes, RNA viruses can overcome host antiviral responses and wreak havoc on the cells they infect. Some manage to evade host antiviral defences, whereas others elicit an aberrant or disproportional immune response. Both scenarios can result in the disruption of intracellular signalling pathways and significant pathology in the host. Systems-biology approaches are increasingly being used to study the processes of viral triggering and regulation of host immune responses. By providing a global and integrated view of cellular events, these approaches are beginning to unravel some of the complexities of virus–host interactions and provide new insights into how RNA viruses cause disease.
Similar content being viewed by others
Main
Viruses can have a devastating effect despite their small genomes. All RNA viruses encode proteins that are essential for structural components and replication, and most encode proteins that function to circumvent host antiviral responses1,2,3. This limited number of proteins is sufficient to ensure the entry, replication and subsequent spread of the virus. However, viruses do not self-propagate and depend on various host-cell functions to complete their life cycle. The processes of viral entry, the triggering and regulation of the host antiviral response and subsequent viral replication together result in an intricate series of interactions between virus and host. Much can be learnt about the nature and complexities of these interactions by global profiling of the transcriptional changes in host cells that occur during viral infection (Box 1).
In this Review, we discuss how functional genomic and systems-biology approaches are contributing to our understanding of interactions between RNA viruses and the host, of viral pathogenesis and of host immunity to infection. Rather than providing a comprehensive literature review, we present examples of how these approaches are providing insight into the interaction of viruses with innate immune defence mechanisms, the evaluation of therapeutics that target these pathways and the crucial balance between protective immune responses and immunopathology. In addition, we describe how genomic approaches are being applied to vaccine evaluation and design, and how these approaches can be combined with other high-throughput technologies to provide an improved and integrated systems-biology view of virus infection.
Although genomic approaches are being used to study a wide variety of viruses, we highlight the current literature through discussion of a select few. Among these is influenza virus, for which the looming threat of a new pandemic and concerns regarding therapeutic and vaccine preparedness have stimulated exciting new research efforts. We also review findings relating to hepatitis C virus (HCV) infection, for which genomic analyses are being used to shed light on the response of patients to treatment with type I interferons (IFNs) and the relationship between HCV replication and liver disease. In addition, we highlight studies of West Nile virus, severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and Ebola virus, all of which have revealed previously undescribed strategies used by these viruses to regulate innate immunity. Finally, we discuss how genomic approaches are being applied to vaccine evaluation and how genomics is being combined with other high-throughput approaches to provide a systems-biology view of virus–host interactions.
Viruses and innate immunity
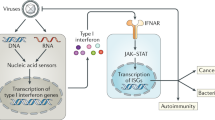
A variety of cellular signalling networks have evolved in host cells to detect and respond to viral infection. One area in which genomics-based analyses are being put to particularly good use is in shedding new light on the components of innate antiviral defence mechanisms and the viral strategies used to overcome them. In this section, we review recent studies in which genomic approaches have been used to provide new information on how viruses trigger and regulate innate immune pathways, and to evaluate the use of type I IFN-based therapy as a means to enhance the innate immune response to HCV.
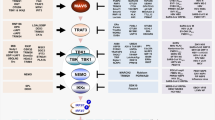
Viral triggering of innate immunity. Mammalian cells have specialized proteins that are responsible for the recognition of virus infection, and other proteins that elicit responses to combat the invading virus. The antiviral response is triggered when host pathogen-recognition receptors (PRRs) are engaged by pathogen-associated molecular patterns (PAMPs) in viral proteins and nucleic acids (reviewed in Refs 4, 5). PRRs that function in virus recognition include the cytosolic double-stranded RNA helicases retinoic-acid-inducible gene I (RIG-I) and MDA5 (melanoma differentiation-associated gene 5) and certain Toll-like receptors (TLRs) that are present on the cell surface or in endosomal membranes. After binding to viral PAMPs, PRRs initiate intracellular signalling cascades that result in the activation of transcription factors, including IFN-regulatory factors (IRFs) and nuclear factor-κB (NF-κB). These transcription factors in turn regulate the expression of hundreds of genes, such as IFNs and IFN-stimulated genes (ISGs)6,7, and pro-inflammatory cytokines and chemokines that are involved in the orchestration of the adaptive immune response (Fig. 1).
Pathogen-associated molecular patterns (PAMPs) in viral proteins and nucleic acids are recognized by cellular pathogen-recognition receptors (PRRs) that include RIG-I (retinoic-acid-inducible gene I), MDA5 (melanoma differentiation-associated gene 5) and certain Toll-like receptors (TLRs). PRR–PAMP interactions trigger signalling cascades that result in the activation of transcription factors, including interferon (IFN)-regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), which induce the production of type I IFNs, IFN-stimulated genes (ISGs) and pro-inflammatory cytokines and chemokines. The specific process differs between antigen-presenting cells, in which both the TLR pathway and the RIG-I or MDA5 pathway are operative, and other cell types, in which only the RIG-I or MDA5 pathway is present. Activation of PRR signalling induces an antiviral state in all cell types, and in antigen-presenting cells it can also induce the production of pro-inflammatory cytokines and chemokines. This normally results in an innate antiviral response that controls infection until it is resolved by the adaptive immune response. However, some viruses, such as the 1918 pandemic influenza virus, elicit an aberrant or disproportional response that results in immunopathology. Alternatively, viruses that suppress the type I IFN response can subvert the mechanisms of innate surveillance and diminish the potential adaptive immune response, resulting in a chronic infection. For vaccine strategies, the best induction of a broad adaptive immune response might require some degree of type I IFN response in the initial stages of infection. DCs, dendritic cells; dsRNA, double-stranded RNA; IFNAR, IFNα receptor; IL, interleukin; IPS1, IFNB-promoter stimulator 1; OAS, 2′,5′-oligoadenylate synthetase; PKR, protein kinase R; ssRNA, single-stranded RNA; STAT, signal transducer and activator of transcription; TAP1, transporter associated with antigen processing 1; TNF, tumour-necrosis factor.
One way in which gene-expression profiling has been used to examine this aspect of the antiviral response is through the use of mouse embryonic fibroblasts deficient in RIG-I or MDA5. A recent study demonstrated that West Nile virus infection of wild-type cells led to the induction of IRF3 target genes and ISGs, including several subtypes of IFNα (Ref. 8). This was followed by a second phase of IFN-dependent antiviral gene expression that occurred at a later stage of infection. By contrast, cells lacking RIG-I had delayed or inhibited initial and secondary gene-expression responses to the virus, indicating that RIG-I has an essential but not exclusive role in initiating innate immune responses to West Nile virus (Fig. 2). The additional deletion of MDA5 in these cells was found to further block their ability to respond to infection, indicating that the host immune response to West Nile virus also involves MDA5. This is a noteworthy finding, as previous studies suggested that RIG-I and MDA5 recognized a specific subset of viruses, rather than acting cooperatively as found in the response to West Nile virus9.
Genomic analyses using cells that lack RIG-I (retinoic-acid-inducible gene I) show the requirement for this pathogen-recognition receptor in the induction of interferon-regulatory factor 3 (IRF3) target genes and interferon-stimulated genes (ISGs) by West Nile virus and influenza virus. a | The infection of RIG-I-deficient cells by West Nile virus results in the delay and partial inhibition of ISG expression. Deletion of MDA5 (melanoma differentiation-associated gene 5) further blocks the response to infection (not shown), indicating that the response to West Nile virus also involves MDA5. b | By contrast, the infection of RIG-I-deficient cells by influenza virus results in a near complete inhibition of ISG expression that is not further blocked by the absence of MDA5, suggesting that MDA5 does not mediate influenza virus-induced gene-expression changes. PAMPs, pathogen-associated molecular patterns. Images generated from data in Refs 8, 10.
The role of RIG-I in the response to influenza virus infection has also been assessed10. Similar to West Nile virus, genomic analysis of influenza virus-infected wild-type and RIG-I-deficient mouse embryonic fibroblasts revealed that RIG-I is necessary for the type I IFN response to this virus (Fig. 2). In RIG-I-deficient cells, influenza virus fails to elicit the expression of IFNβ and of many ISGs, including key antiviral mediators such as IRF3, STAT1 (signal transducer and activator of transcription 1), IFIT1 (IFN-induced protein with tetratricopeptide repeats 1; also known as ISG56) and ISG54 (also known as IFIT2). This study also showed that, unlike during infection with West Nile virus, MDA5 does not function as a secondary mediator of the response to infection with influenza virus10. Important next steps in these studies will be to compare the profiles of genes induced by each of these viruses — and to determine whether some genes are specific for RIG-I or MDA5 signalling — and to begin to define the involvement of these genes in innate immunity. Although this biological validation process will be necessary to follow-up genomic analyses, few studies so far have included such experiments.
Functional genomic analyses have also been helpful in elucidating the complex transcriptional events triggered following TLR signalling. TLRs are expressed by various immune cells, including macrophages, dendritic cells and lymphocytes, and a subset of these receptors are involved in viral recognition. So far, genomic studies have largely focused on the analysis of macrophages treated with TLR ligands, such as lipopolysaccharide (LPS; a component of the cell wall of Gram-negative bacteria) or polyinosinic–polycytidylic acid (a synthetic mimic of viral double-stranded RNA, dsRNA)11,12,13.
To obtain a comprehensive view of the transcriptional programmes that are induced by TLR activation, Elkon et al. used a computational approach to analyse gene-expression data sets derived from four studies in which human or mouse macrophages were stimulated with pathogen-mimetic agents that engage various TLRs14. This analysis identified one transcriptional profile that is universally activated by all TLRs and a second profile that is specific to both TLR3 (which specializes in the recognition of viral dsRNA) and TLR4 (which recognizes envelope components of viruses and cell-surface components of bacteria (such as LPS)). A computational analysis of promoter sequences identified NF-κB as the key regulator of the universal response, which occurs early after TLR stimulation, and the IFN-stimulated response element (ISRE) as the key component of the TLR3 and TLR4 response, which is induced after the NF-κB response. This computational approach provided additional knowledge regarding the kinetics of the TLR3 and TLR4 response, the regulatory circuitry involved and the identity of the genes activated in both the universal and TLR3- and TLR4-mediated responses. Although these studies have provided considerable information regarding the genes activated downstream of TLR activation, it will be advantageous to extend genomic analyses in the context of viral infection using cells lacking the expression of specific TLRs.
Viral regulation of innate immunity. The ability of a virus to establish an infection depends, at least to some extent, on its ability to block the host innate immune response or to modulate the activity of antiviral effector proteins. HCV is one example of a virus that has devised a means to block the initial triggering of the host innate immune response. Several studies have shown that the HCV NS3–NS4A serine protease blocks the TLR3-dependent activation of IRF3 (Refs 17, 19). This is achieved by NS3–NS4A-mediated cleavage of TRIF (Toll/interleukin-1 (IL-1) receptor-domain-containing adaptor protein inducing IFNβ), an adaptor protein that links TLR3 to kinases that are responsible for activating IRF3 and NF-κB17,19. HCV also inhibits the ability of RIG-I to activate IRF3 (Refs 15, 16, 18, 20), which is achieved through NS3–NS4A-mediated cleavage of IPS1 (IFNB-promoter stimulator 1; also known as VISA, CARDIF, MAVS), a recently identified RIG-I adaptor protein21,22,23,24,25.
In light of these findings, it is both perplexing and paradoxical that virtually all gene-expression profiling carried out using HCV-infected tissue shows the induction of ISG expression, including IRF3 target genes26,27,28,29,30,31,32,33,34,35,36. The induction of ISG expression is observed in liver tissue from HCV-infected patients30,32,37 and during the initial host response in acutely infected chimpanzees26,28, and is a major part of the transcriptional response to HCV infection in the chimeric SCID-Alb/uPA mouse model33. This poses an interesting question about the source of both type I IFNs and ISG expression. It is possible that ISGs are mainly expressed in uninfected hepatocytes and are induced in response to exogenous type I IFN released from adjacent HCV-infected cells. Alternatively, it has been suggested that T cells and plasmacytoid dendritic cells that infiltrate the liver are a possible source of hepatic type I IFNs37. Although this is possible, it is relevant to note that HCV infection in the SCID-Alb/uPA mouse model is also associated with the induction of hepatic ISG expression in the absence of these immune cell types33.
Other genomic studies have revealed examples of highly virulent viruses that are relatively successful at inhibiting ISG expression. Perhaps the best example is a characterization of the host transcriptional response of human liver cells infected with filoviruses38. This study demonstrated the marked suppression of genes in key innate antiviral pathways, including those mediated by IRF3. Interestingly, this study also suggested a correlation between the antagonism of the type I IFN response and filovirus virulence. Highly virulent viruses, such as Zaire Ebola virus and Marburgvirus, inhibit the expression of most ISGs that are induced in uninfected IFN-treated cells. By contrast, the relatively non-pathogenic Reston Ebola virus is less inhibitory and induces the expression of more than 20% of these genes. The suppression of the type I IFN response by the pathogenic viruses is associated with more rapid viral spread and higher rate of viral replication than that observed during Reston Ebola virus infection.
A comparable trend was seen in a study evaluating the host transcriptional response and inflammation in the brains of mice infected with rabies virus39. This study revealed that infection with an attenuated virus results in both inflammation and the induction of expression of key ISGs. However, these events are either absent or diminished during infection with a highly pathogenic rabies virus. On the basis of results with filoviruses, it would follow that attenuation of the type I IFN response would be associated with higher viral replication and spread in the case of pathogenic infection with rabies virus; however, this was not measured in the study. Similarly, infection with highly virulent pseudorabies virus suppressed the induction of a subset of ISGs, even in type I IFN-treated cells40. Together, these data suggest that the virulence of acute, highly pathogenic viruses is at least partially related to their ability to suppress the host antiviral response, which seems to allow higher levels of viral replication.
Suppression of innate immunity and persistent infection. Evidence discussed in this Review suggests that suppression of elements of the innate immune response enables extensive viral replication and increased pathogenesis. Does the converse hold true for a virus such as HCV, which typically establishes a persistent infection characterized by mild (or slowly progressing) disease? Some evidence suggests that this might be the case; for example, studies using the chimeric SCID-Alb/uPA mouse model indicate that an attenuated type I IFN response is associated with higher levels of intrahepatic HCV replication together with a greater induction of lipid metabolism and oxidative-stress genes, which have the potential to cause cytopathic effects33.
Similarly, gene-expression profiling of serial liver biopsies obtained from patients that had received an HCV-infected liver transplant shows that rapid progression of fibrosis following transplantation is associated with the suppression of genes involved in the type I IFN response, antigen presentation and the cytotoxic T-cell response30. Although in these studies the apparent defect in the host antiviral response is probably related to host genetics rather than viral factors, the concept that a defective innate immune response correlates with enhanced pathogenesis is still evident. It is possible that the selective pressures on persistent viruses never resulted in a need for a complete subversion of host innate antiviral responses, so such viruses use these responses to limit their replication to a level that does not significantly affect the normal functions of the host cell. Conversely, acute viruses, such as filoviruses, highly pathogenic influenza virus and rabies virus, seem to have evolved to antagonize these responses following cell entry to allow immediate, high levels of replication, which subsequently facilitate virus spread and transmission.
Innate immunity as a target for therapeutic intervention. Given the importance of the innate immune response in regulating virus infection, there is considerable interest in enhancing or modulating this response for therapeutic benefit. One role for genomics in this area is assisting in the evaluation of type I IFN treatment of HCV infection. Combination therapy with IFNα and the antiviral drug ribavirin results in virus clearance in only ∼50% of individuals infected with HCV genotype 1 and ∼80% of individuals infected with HCV genotypes 2 or 3 (Refs 41,42,43,44). As IFNα is the only approved treatment for chronic HCV, there is strong interest in improving this therapy, in understanding the molecular mechanisms that underlie treatment failure and in identifying markers to accurately predict a patient's response to treatment (that is, responders or non-responders).
Several groups that have used transcriptional profiling of patient hepatic tissue to address these issues have found that higher levels of expression of ISGs before treatment are associated with treatment failure. For example, Chen et al. carried out microarray experiments on pretreatment liver tissue obtained from a cohort of 31 patients with chronic HCV infection who subsequently underwent IFNα and ribavirin therapy45. This analysis identified a set of 18 genes, many of which are known ISGs; in general these genes were more highly induced in the livers of patients that did not respond to therapy. Although the authors suggest that this set of genes could therefore be used to predict the response to therapy, it remains to be determined whether they can be used to accurately predict the response in other patient cohorts. Similarly, Feld et al. showed that non-responders have significantly higher intrahepatic pretreatment expression levels of ISGs than patients who respond to type I IFN therapy46. Although these studies are intriguing, it is still unclear whether there is a causal relationship between higher pretreatment levels of ISGs and therapy failure. Other factors, such as viral quasispecies diversity, may also be important.
Owing to the technical and ethical issues of obtaining sufficient liver material for gene-expression studies, investigators have also used peripheral-blood mononuclear cells (PBMCs) to evaluate the response to treatment47,48. An example is ViraHepC, a multicentre study designed to define the differences in response rates among Caucasian and African Americans and to identify host and viral parameters associated with a lack of response to treatment48. Overall, this study showed that, during the first 28 days of treatment, a lower level of induction of known ISGs is associated with non-responsiveness to type I IFN treatment. However, in many cases, these differences are not strikingly dissimilar between responders and non-responders. The implication of such minor differences with respect to antiviral function is uncertain and the feasibility of using them for predicting a patient's response is questionable. In addition, analyses using PBMCs should be interpreted with caution, as a recent study showed that the transcriptional response to type I IFN treatment is significantly different in the blood and the liver of HCV-infected chimpanzees, presumably owing to the absence of HCV replication in PBMCs49. Although it has not yet been evaluated, this will almost certainly hold true for humans as well.
An alternative mechanism of a failed response to type I IFN treatment could involve the induction of genes associated with IFN inhibitory pathways46. Walsh et al. found significantly increased intrahepatic expression of the gene encoding suppressor of cytokine signalling 3 (SOCS3) in patients who did not respond to type I IFN treatment50. Enhanced intrahepatic SOCS3 expression is also thought to contribute to the non-responsiveness of HCV-infected chimpanzees to type I IFN therapy51. However, a separate evaluation of 21 patients for intrahepatic SOCS3 mRNA expression before antiviral therapy actually found higher levels of expression in those patients who went on to respond successfully to type I IFN treatment51. Therefore, the relationship between treatment failure and induction of type I IFN inhibitory pathways is currently less clear than that between higher pretreatment levels of expression of ISGs and treatment failure.
Innate immune protection versus immunopathology. There are still surprisingly few answers to the fundamental question of how virus infection results in disease pathology. Although the mechanisms are certain to be different for each virus, a common theme is that there is a crucial balance between protective immune responses and immunopathology52,53. Although the innate immune response is designed to target and eliminate invading pathogens, genomic analyses have indicated that some viruses, such as the highly virulent influenza virus that was responsible for the 1918 pandemic, elicit aberrant or disproportional innate immune responses that may also harm the host.
The 1918 influenza virus pandemic (known as the Spanish Flu) killed as many as 50 million people worldwide54, and several studies have begun to provide clues to what made this virus so deadly (reviewed in Refs 55,56,57). Although genomic analyses have previously been carried out using engineered viruses containing one or more genes from the 1918 pandemic virus58,59, a major advance in the ability to study this virus came from its reconstruction based on nucleotide sequence information60. Genomic analyses of lung or bronchial tissue derived from mice or macaques that were infected with the reconstructed 1918 virus indicate how the beneficial role of the innate immune response can be tipped towards immunopathology.
Mice infected with the reconstituted 1918 influenza virus show severe pulmonary pathology and an increased and accelerated transcriptional activation of immune-response genes61. This includes a marked activation of genes associated with pro-inflammatory and cell-death pathways by 24 hours after infection (Fig. 3), which remain unabated until the death of the animals. This response is in contrast to the less dramatic and delayed host immune responses (and less severe disease pathology) in mice that were infected with influenza viruses containing only subsets of genes from the 1918 virus, including the haemagglutinin (HA) and non-structural protein (NS) genes, or the HA, neuraminidase (NA), matrix (M) and nucleoprotein (NP) genes. These findings suggest that enhanced pro-inflammatory and cell-death responses can contribute to severe immunopathology.
In a mouse infection model, contemporary and 1918 pandemic influenza viruses each trigger an innate immune response that includes the expression of nuclear factor-κB (NF-κB) and interferon-regulatory factor 3 (IRF3) target genes. However, the gene-expression response triggered by the contemporary virus is moderate and transient and accompanied by only mild clinical symptoms. The gene-expression response to the 1918 virus is aberrantly high and sustained and may contribute to the severe clinical symptoms, including alveolitis, haemorrhage and neutrophil infiltration, that are observed in animals infected with this virus. This disproportional innate immune response and resulting immunopathology could also be the cause of the increased severity of symptoms observed in people during the 1918 pandemic. Images reproduced, with permission, from Nature Ref. 61 © 2006 Macmillan Publishers Ltd. All rights reserved.
An additional study that evaluated the host response to the 1918 influenza virus using a cynomologus macaque (Macaca fascicularis) infection model produced similar results62. In macaques, the 1918 virus replicates to high levels and spreads rapidly throughout the respiratory tract of infected animals, causing severe lung damage and the massive infiltration of immune cells throughout the course of infection. Functional genomic analyses of bronchial tissue revealed that the 1918 virus triggers the aberrantly high and sustained expression of numerous genes involved in the innate immune response, including pro-inflammatory cytokines and chemokines. Although the timing of the response is somewhat different, the increased and sustained host response in macaques that were infected with the 1918 virus is similar to that observed in mice.
These studies reveal similarities and differences in the host response to contemporary and 1918 pandemic influenza virus infection. First, contemporary and 1918 viruses each trigger an innate immune response that includes the expression of NF-κB and IRF3 target genes, which is expected to occur if the virus triggers the RIG-I pathway in infected respiratory cells. Second, both viruses trigger a robust cytokine response that probably attracts immune-cell infiltration to infected tissues. Unlike contemporary virus strains, in which the early response to infection is resolved, the innate immune response triggered by the 1918 virus is characterized by a strong and sustained induction that is associated with massive tissue damage and death of the infected animal. However, in preliminary genomic analyses carried out with lung tissue from macaques that were infected with avian H5N1 viruses, we have found that there are significant differences in the regulation of antiviral responses by the 1918 pandemic and H5N1 viruses (J. C. Kash and M.G.K., unpublished observations). Therefore, there may be differences in the ways in which highly pathogenic influenza viruses regulate the innate immune response and cause disease.
The enhanced pathogenicity of the 1918 and H5N1 influenza viruses might be attributed to distinct components of their genomes. Although much emphasis has been placed on the NS1 protein of the 1918 virus acting as an inhibitor of the type I IFN response, recent evidence suggests that the viral proteins PB1 (a polymerase), HA and NA contribute to its pathogenicity63. Likewise, the polymerases of H5N1 viruses have been linked to increased viral pathogenesis64, suggesting that the increased pathogenesis of these viruses may be related to their replicative fitness.
Another respiratory virus, SARS-CoV, has emerged recently and has caused great concern among the public health and research communities. It has been suggested that disease pathology associated with SARS-CoV is caused by a disproportional immune response, illustrated by increased levels of pro-inflammatory cytokines and chemokines65,66,67. Studies carried out in our laboratory have combined the use of functional genomics with a cynomologus macaque infection model to study the host response to this virus68. We observed that SARS-CoV-infected macaques show a strong increase in the expression of innate immune response genes early after infection and that this response wanes after 4 days. Conversely, genes that are induced later in infection tend to be involved in the cell cycle and in cell repair. None of the animals used in this study succumbed to infection, and SARS-CoV-induced pathology in these macaques resembled the pathological changes seen in the majority of human patients with SARS who recover from the disease68. Unlike the findings of the 1918 pandemic influenza virus study, these data suggest that early immune responses to SARS-CoV infection are productive and enable the host to properly fight the virus, allowing a return to cellular homeostasis. However, in the 10% of human infections in which SARS-CoV infection is fatal (mostly in the elderly), it is possible that the timing or magnitude of the response results in immunopathology. Studies using aged macaques might help to address this possibility.
Viruses such as SARS-CoV, H5N1 influenza virus and 1918 influenza virus are all zoonotic infections, in which a virus that was adapted to another host was transferred to humans. Because the type I IFN response is somewhat different in different hosts, it is possible that these viruses, which have adapted to their normal animal hosts, elicit an aberrant response when infecting a human host in which adaptation has not occurred, resulting in immunopathology. This possibility also raises the question of how appropriate the various animal infection models (such as mice and macaques) are for the understanding of human pathogenesis. As reviewed elsewhere56, there are both advantages and disadvantages associated with different animal models, and it is important to keep in mind that responses observed using an animal model may not always accurately reflect the response in humans.
Genomics in vaccine evaluation and design
Genomic information and high-throughput technologies are beginning to have an impact on the field of vaccine development, but the main focus has been directed towards identifying important conserved features of pathogens that could serve as immunogens and characterizing host genotypes associated with strong protective responses69,70,71 (Box 2). In recent years, it has become evident that the type I IFN response has a significant role in the development of the adaptive immune response. This commences with the influence of type I IFNs on the activation, maturation and migration of dendritic cells72,73. The development of the antibody response is also enhanced by type I IFNs through the direct effect of IFN on B cells and on the priming or function of CD4+ T helper cells74. There is now also evidence that type I IFNs act directly on CD8+ T cells to promote clonal expansion and indirectly by stimulating cross-priming by antigen-presenting cells that have engulfed infected cells to acquire antigen75,76,77. So, viruses that suppress the type I IFN response not only subvert the mechanisms of innate surveillance, but also diminish the potential adaptive immune response that could mediate viral clearance or establish a quiescent, non-pathogenic state. For vaccine strategies, the implication is then that the best induction of a broad adaptive immune response will require some degree of type I IFN response in the initial stages.
Animal models. We have used functional genomics to evaluate a live influenza virus vaccine in a macaque model, in which attenuation of the virus was accomplished by truncation of the gene encoding NS178. This modification eliminates or reduces the ability of the NS1 protein to antagonize type I IFN production79 and, in mouse and swine models, such attenuated live viruses are immunogenic and protective80,81. Gene-expression profiling of tracheal and bronchial epithelial cells from macaques immunized with the NS1-truncated virus show clear evidence of a robust type I IFN response. Compared with immunization with a traditional killed-virus vaccine, the attenuated live-virus-vaccine group had higher antibody titres before and after challenge and a broader range of influenza virus-specific T-cell responses. Following challenge with infective virus, the protection afforded by the attenuated live-virus vaccine was evident by the limited viral replication and minor pathology observed in treated animals. In addition, gene-expression profiles of lung tissue from animals that received the attenuated live-virus vaccine show less upregulation of innate and pro-inflammatory response genes compared with animals immunized with the killed-virus vaccine or untreated animals. At the same time, the transcriptional profiles for the attenuated live-virus-vaccine animals showed a stronger induction of genes that are associated with B-cell and T-cell responses.
The general picture overall is that the truncated-NS1-containing influenza virus vaccine undergoes minimal replication but induces sufficient type I IFNs to galvanize the adaptive immune response, leaving the host in a state of adaptive preparedness after just one immunization. The early induction of type I IFNs in response to the truncated-NS1-containing vaccine might be especially important in the local B-cell response that is crucial for viral clearance. A relevant observation in this regard is that early stimulation of the respiratory-tract B cells (within 48 hours of influenza virus infection) was shown to be strongly driven by virus-induced type I IFNs82,83.
Human studies. At present, there are only limited examples in which gene-expression profiling applied to vaccine design supports a picture consistent with that described above for the influenza virus model. The standards for prevention of measles and yellow fever are immunizations with attenuated live-virus vaccines. To assess the impact of infection on primary target cells, gene-expression profiling was carried out in tissue-culture systems comparing wild-type and vaccine strains. For both measles and yellow fever, it was clear that the attenuated vaccine strains led to a greater induction of the type I IFN response than the pathogenic wild-type virus84,85. Although in the case of measles virus this disparity in the IFN response has previously been shown by serological techniques86, expression analysis indicated that the antagonism of the response by the wild-type virus originated at the level of transcription. This early induction of the type I IFN response was also evident in microarray studies examining chimeras of the yellow fever vaccine strain that were devised as attenuated live-virus vaccines against other flaviviruses such as Dengue virus87. This contrasted with the low-level induction of type I IFNs by Dengue virus infection as seen by expression profiling using infection of primary cells or macaque disease models87,88.
It is interesting to note that the measles and yellow fever vaccine strains are attenuated by passage in cells from other species. Therefore, with suitable molecular understanding, the ability of some viruses to induce type I IFNs might be optimized by directed molecular techniques, as was done for the truncated-NS1 influenza virus strain. As an alternative, one might consider using recombinant type I IFNs as vaccine adjuvants instead of inducing them with the vaccine constituents89, but at our present level of understanding, these approaches have yet to prove clinically tenable90.
Functional genomics for the evaluation of immunological memory. Functional genomic studies have been more equivocal in assessing the significance of type I IFN production during the immunological memory response. In the aforementioned macaque influenza virus study, animals receiving the attenuated live-virus vaccine showed upregulation of type I IFN pathways in tracheobronchial cells 2 days after challenge, and this coincided with the development of a strong memory response78. This type I IFN induction seems to be weaker than that observed at the corresponding time after the primary exposure to the vaccine, but is far lower than the type I IFN induction observed after challenge of animals receiving the killed-virus vaccine or of naive animals. This would suggest some role of this innate pathway in stimulating immunological recall.
In contrast to this, examination of transcriptional profiles observed shortly after rechallenge of human PBMCs from individuals previously immunized against influenza virus are more in accord with early production of IFNγ, possibly arising from antigenic stimulation of memory cells91. Dhiman et al. also did not see evidence of a type I IFN response in a microarray study of whole blood taken from individuals immunized with measles virus after rechallenge with an attenuated live-virus vaccine strain, although genes associated with lymphocyte activation and survival were upregulated92. It could be considered that technical issues might hamper the relevance of these studies in assessing the role of type I IFNs in the memory response. In the case of the first study91, PBMCs are not a primary target of influenza virus, so virus internalization might have been inefficient and a type I IFN response might have been poor. In the measles study92, the earliest time point examined was 7 days after rechallenge rather than early, when the type I IFN response would be expected to be strongest. Therefore, further functional genomic experiments, with appropriately designed models, are required to address whether an early innate immune response is a key stage in triggering immunological memory.
Future prospects
Functional genomics has proven to be a highly efficient method for providing broad views of the host response in studies of virus–host interactions. As we have discussed, these techniques have revealed the activation or repression of innate immune signalling pathways, crosstalk between pathways, the timing and magnitude of the immune response and, depending on the experimental system, the degree to which the immune response varies among individuals.
Conversely, functional genomics has been less effective in pinpointing the role of specific host genes in the antiviral response or, somewhat surprisingly, in identifying previously undiscovered genes and pathways that are important in the infection process, despite this being one of its early goals93. Indeed, the early assumption that functional genomics would provide quick answers to the complexities of virus–host interactions has proved naive. How then can greater benefits be gained from using functional genomics to study virus–host interactions? Rather than being used as a singular approach, the future of functional genomics in virology will be in the integration of genomic data with data derived from other high-throughput technologies (Fig. 4).
The benefits of functional genomics will be further enhanced by integrating genomic data with data derived from other high-throughput technologies. The potential information and biological insights provided by these technologies are shown. Together, these approaches will help to provide a systems-biology view of virus–host interactions that spans the flow of biological information from DNA (genetics) to mRNA (genomics) to protein (proteomics) to protein function (immunomics).
The obvious complementary approach to functional genomics is proteomics, which will provide much needed information regarding the correlation of gene expression with protein abundance94,95,96. Our group has begun to integrate genomic and proteomic data to better understand the host response to influenza virus infection97. Other possibilities for data integration are also beginning to unfold. For example, microRNAs, which regulate both transcription and translation, might have an important role in mediating virus–host interactions98. The discovery of microRNAs in certain large DNA viruses, such as herpesvirus, suggests that some viruses may encode microRNAs to regulate cellular functions99. In addition, immunomic strategies (Box 2) will provide additional opportunities to interrogate the host immune response; screens using small interfering RNAs are currently being combined with genomic data to identify specific cellular proteins that are used by viruses during infection100,101,102. Together with virology, clinical and pathology data, this integrated set of information might provide the systems-biology view that will be needed to clearly understand the role of specific host genes and pathways involved in the development of immunity or disease after virus infection.
Another use for genomics that will no doubt expand is expression quantitative trait loci (eQTL) mapping103. The combination of global gene-expression data with eQTL mapping provides greater power in elucidating complex genetic traits in addition to providing insights into specific genes or mutations that might be responsible for the trait in question. This approach is currently being used to better understand the genetic basis for various disease conditions in mice104,105,106, and it is likely that it will also be useful in increasing our understanding of virus–host interactions. For example, using recombinant inbred strains of mice derived from parental strains that react differently to infection with a given virus, it should be possible to use eQTL mapping to determine chromosomal locations for potential trait-contributing factors and highlight genes of interest for the trait.
With this increased level of complexity, however, it will be important to work closely with the bioinformatics and computational-modelling communities, and to make best use of the sophisticated bioinformatics tools, data-mining schemes and mathematical-modelling strategies that are continually being developed107,108. It might also be necessary to take a step back to simpler experimental systems (such as cell-culture models) to dissect cellular events before moving on to more complex in vivo models. The use of combined computational approaches that can account for gene-regulatory networks and cell-to-cell interactions will also facilitate the move to whole animal physiological modelling.
Functional genomics is clearly providing advances in our understanding of virus–host interactions, and the evolution to an integrated systems-biology approach holds even greater promise for the field. In addition to providing new insights into viral pathogenesis and host immunity, this approach provides a host-oriented antiviral discovery paradigm with the potential for discovering new targets for broad-spectrum antiviral therapies109 and for improving vaccine evaluation and design. We are optimistic about continuing advancements in the technologies and computational methods used to study virus–host interactions and in improved capabilities to identify, characterize and circumvent the strategies used by viruses to outsmart their long-suffering hosts.
Note added in proof
Recent studies have shown that RIG-I preferentially recognizes single-stranded RNA (ssRNA) with polyU motifs, whereas MDA5 recognizes long dsRNA molecules. These differences might help to explain the differential recognition and innate immune signalling induced by different RNA viruses117,118,119.
References
Haller, O. & Weber, F. Pathogenic viruses: smart manipulators of the interferon system. Curr. Top. Microbiol. Immunol. 316, 315–334 (2007).
Roy, C. R. & Mocarski, E. S. Pathogen subversion of cell-intrinsic innate immunity. Nature Immunol. 8, 1179–1187 (2007).
Katze, M. G., He, Y. & Gale, M. Jr. Viruses and interferon: a fight for supremacy. Nature Rev. Immunol. 2, 675–687 (2002).
Saito, T. & Gale, M. Jr. Principles of intracellular viral recognition. Curr. Opin. Immunol. 19, 17–23 (2007).
Thompson, A. J. & Locarnini, S. A. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol. Cell Biol. 85, 435–445 (2007).
de Veer, M. J. et al. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69, 912–920 (2001).
Der, S. D., Zhou, A., Williams, B. R. G. & Silverman, R. H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl Acad. Sci. USA 95, 15623–15628 (1998).
Fredericksen, B., Keller, B., Fornek, J., Katze, M. G. & Gale, M. Jr. Establishment and maintenance of the innate antiviral response to West Nile virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 82, 609–616 (2007). In this study, genomic analyses show that RIG-I and MDA5 operate cooperatively to establish an antiviral state and mediate an IFN amplification loop that supports immune effector gene expression during West Nile virus infection.
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Loo, Y. M. et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82, 335–345 (2008).
Gilchrist, M. et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 (2006).
Sharif, O., Bolshakov, V. N., Raines, S., Newham, P. & Perkins, N. D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 8, 1 (2007).
Nau, G. J. et al. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl Acad. Sci. USA 99, 1503–1508 (2002).
Elkon, R., Linhart, C., Halperin, Y., Shiloh, Y. & Shamir, R. Functional genomic delineation of TLR-induced transcriptional networks. BMC Genomics 8, 394 (2007).
Foy, E. et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300, 1145–1148 (2003).
Foy, E. et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl Acad. Sci. USA 102, 2986–2991 (2005).
li, K. et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl Acad. Sci. USA 102, 2992–2997 (2005).
Sumpter, R. Jr et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699 (2005).
Ferreon, J. C., Ferreon, A. C., li, K. & Lemon, S. M. Molecular determinants of TRIF proteolysis mediated by the hepatitis C virus NS3/4A protease. J. Biol. Chem. 280, 20483–20492 (2005).
Breiman, A. et al. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKε. J. Virol. 79, 3969–3978 (2005).
Loo, Y. M. et al. Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc. Natl Acad. Sci. USA 103, 6001–6006 (2006).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunol. 6, 981–988 (2005).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 (2005).
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Bigger, C. B., Brasky, K. M. & Lanford, R. E. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75, 7059–7066 (2001).
Bigger, C. B. et al. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J. Virol. 78, 13779–13792 (2004).
Su, A. I. et al. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl Acad. Sci. USA 99, 15669–15674 (2002).
Smith, M. W. et al. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology 38, 1458–1467 (2003).
Smith, M. W. et al. Gene expression patterns that correlate with hepatitis C and early progression to fibrosis in liver transplant recipients. Gastroenterology 130, 179–187 (2006). This study uses gene-expression profiling of serial liver-biopsy samples from patients that had received a liver transplant to demonstrate that rapidly progressive fibrosis is associated with an impaired immune response, as indicated by a lack of induction of genes associated with the IFN-mediated antiviral response, antigen presentation and cytotoxic T-cell response.
Lederer, S. L. et al. Distinct cellular responses differentiating alcohol- and hepatitis C virus-induced liver cirrhosis. Virol. J. 3, 98 (2006).
Walters, K. A. et al. Identification of a specific gene expression pattern associated with HCV-induced pathogenesis in HCV- and HCV/HIV-infected individuals. Virology 350, 453–464 (2006).
Walters, K. A. et al. Host-specific response to HCV infection in the chimeric SCID-beige/Alb-uPA mouse model: role of the innate antiviral immune response. PLoS Pathog. 2, 591–601 (2006). Using the SCID-Alb/uPA mouse model of HCV infection, this study shows that host genetics have an impact on the nature of the initial innate antiviral response to HCV infection, which in turn determines the extent of virus-mediated effects on host gene expression.
Bieche, I. et al. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology 332, 130–144 (2005).
Lau, D. T., Luxon, B. A., Xiao, S. Y., Beard, M. R. & Lemon, S. M. Intrahepatic gene expression profiles and α-smooth muscle actin patterns in hepatitis C virus induced fibrosis. Hepatology 42, 273–281 (2005).
Helbig, K. J., Lau, D. T., Semendric, L., Harley, H. A. & Beard, M. R. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology 42, 702–710 (2005).
Lau, D. T. et al. Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology 47, 799–809 (2008).
Kash, J. C. et al. Global suppression of the host antiviral response by Ebola and Marburgviruses: increased antagonism of the type 1 interferon response is associated with enhanced virulence. J. Virol. 80, 3009–3020 (2006). This study provides a thorough genomic analysis showing a correlation between antagonism of type I IFN responses and filovirus virulence.
Wang, Z. W. et al. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J. Virol. 79, 12554–12565 (2005).
Brukman, A. & Enquist, L. W. Suppression of the interferon-mediated innate immune response by pseudorabies virus. J. Virol. 80, 6345–6356 (2006).
Thomson, B. J. & Finch, R. G. Hepatitis C virus infection. Clin. Microbiol. Infect. 11, 86–94 (2005).
Lindsay, K. L. Introduction to therapy of hepatitis C. Hepatology 36, S114–S120 (2002).
Lau, D. T. et al. 10-Year follow-up after interferon-α therapy for chronic hepatitis C. Hepatology 28, 1121–1127 (1998).
Manns, M. P. et al. Peginterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001).
Chen, L. et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology 128, 1437–1444 (2005). This study compares hepatic gene expression in HCV-infected patients prior to IFN and ribavirin therapy and demonstrates that upregulation of a specific set of IFN-responsive genes predicts non-responsiveness to exogenous therapy.
Feld, J. J. et al. Hepatic gene expression during treatment with peginterferon and ribavirin: identifying molecular pathways for treatment response. Hepatology 46, 1548–1563 (2007). Gene-expression profiling of hepatic tissue obtained prior to and during IFN treatment of HCV-infected patients shows that treatment failure was associated with a higher pretreatment ISG expression and a greater induction of IFN inhibitory pathways.
He, X. S. et al. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology 44, 352–359 (2006).
Taylor, M. W. et al. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from nonresponders to antiviral therapy. J. Virol. 81, 3391–3401 (2007).
Lanford, R. E. et al. Lack of response to exogenous interferon-α in the liver of chimpanzees chronically infected with hepatitis C virus. Hepatology 46, 999–1008 (2007). In this paper, the authors examine the transcriptional response to IFN in the liver and in PBMCs from uninfected chimpanzees and find that the response is largely tissue specific and rapidly downregulated in vivo.
Walsh, M. J. et al. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut 55, 529–535 (2006).
Huang, Y. et al. Defective hepatic response to interferon and activation of suppressor of cytokine signaling 3 in chronic hepatitis C. Gastroenterology 132, 733–744 (2007).
Karupiah, G. & Chaudhri, G. Immunopathogenesis of infectious disease: injury and death from friendly fire. Immunol. Cell Biol. 85, 5 (2007).
Clark, I. A. The advent of the cytokine storm. Immunol. Cell Biol. 85, 271–273 (2007).
Taubenberger, J. K. & Morens, D. M. 1918 Influenza: the mother of all pandemics. Emerg. Infect. Dis. 12, 15–22 (2006).
Ahmed, R., Oldstone, M. B. & Palese, P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nature Immunol. 8, 1188–1193 (2007).
Fornek, J. L., Korth, M. J. & Katze, M. G. Use of functional genomics to understand influenza–host interactions. Adv. Virus Res. 70, 81–100 (2007).
La Gruta, N. L., Kedzierska, K., Stambas, J. & Doherty, P. C. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85, 85–92 (2007).
Geiss, G. K. et al. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl Acad. Sci. USA 99, 10736–10741 (2002).
Kash, J. C. et al. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J. Virol. 78, 9499–9511 (2004).
Tumpey, T. M. et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310, 77–80 (2005).
Kash, J. C. et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443, 578–581 (2006).
Kobasa, D. et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445, 319–323 (2007). Genomic analyses indicate that cynomologus macaques infected with the 1918 influenza virus mount an immune response that is characterized by dysregulation of the antiviral response and that is insufficient for protection. This indicates that atypical host innate immune responses might contribute to lethality.
Pappas, C. et al. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc. Natl Acad. Sci. USA 105, 3064–3069 (2008).
Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840–1842 (2002).
Cameron, M. J. et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 81, 8692–8706 (2007).
Reghunathan, R. et al. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 6, 2 (2005).
Huang, K. J. et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 75, 185–194 (2005).
de Lang, A. et al. Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathog. 3, e112 (2007).
De Groot, A. S. Immunomics: discovering new targets for vaccines and therapeutics. Drug. Discov. Today 11, 203–209 (2006).
Poland, G. A., Ovsyannikova, I. G., Jacobson, R. M. & Smith, D. I. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin. Pharmacol. Ther. 82, 653–664 (2007). This is a good review discussing host genotypic variation and heterogeneity in vaccine immune responses.
Rappuoli, R. Bridging the knowledge gaps in vaccine design. Nature Biotechnol. 25, 1361–1366 (2007).
Asselin-Paturel, C. et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201, 1157–1167 (2005). These authors show the importance of type I IFNs in plasmacytoid dendritic-cell activation and migration using an elegant series of in vivo mouse models that exploited IFNα-receptor-deficient mice and synthetic TLR ligands.
Luft, T. et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161, 1947–1953 (1998).
Le, B. A. et al. Cutting Edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176, 2074–2078 (2006).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Thompson, L. J., Kolumam, G. A., Thomas, S. & Murali-Krishna, K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177, 1746–1754 (2006).
Le, B. A. et al. A role for the transcription factor RelB in IFN-α production and in IFN-α-stimulated cross-priming. Eur. J. Immunol. 36, 2085–2093 (2006).
Baskin, C. R. et al. Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J. Virol. 81, 11817–11827 (2007).
Fernandez-Sesma, A. et al. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80, 6295–6304 (2006).
Ferko, B. et al. Immunogenicity and protection efficacy of replication-deficient influenza A viruses with altered NS1 genes. J. Virol. 78, 13037–13045 (2004).
Vincent, A. L. et al. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine 25, 7999–8009 (2007).
Coro, E. S., Chang, W. L. & Baumgarth, N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176, 4343–4351 (2006).
Chang, W. L. et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178, 1457–1467 (2007).
Bolt, G., Berg, K. & Blixenkrone-Moller, M. Measles virus-induced modulation of host-cell gene expression. J. Gen. Virol. 83, 1157–1165 (2002). Despite a limited technical capacity to measure only 1,176 genes, this early paper is a good demonstration of the power of global transcriptional profiling: the results presaged publications using diverse technologies to characterize other attenuated live-virus vaccines.
Lefeuvre, A. et al. Host-cell interaction of attenuated and wild-type strains of yellow fever virus can be differentiated at early stages of hepatocyte infection. Microbes Infect. 8, 1530–1538 (2006).
Naniche, D. et al. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of α/β interferon production. J. Virol. 74, 7478–7484 (2000).
Deauvieau, F. et al. Innate immune responses in human dendritic cells upon infection by chimeric yellow-fever dengue vaccine serotypes 1–4. Am. J. Trop. Med. Hyg. 76, 144–154 (2007).
Sariol, C. A. et al. Transcriptional activation of interferon-stimulated genes but not of cytokine genes after primary infection of rhesus macaques with dengue virus type 1. Clin. Vaccine Immunol. 14, 756–766 (2007).
Bracci, L. et al. Type I IFN as a vaccine adjuvant for both systemic and mucosal vaccination against influenza virus. Vaccine 24 (Suppl 2), 2–7 (2006).
Chabalgoity, J. A., Baz, A., Rial, A. & Grille, S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 18, 195–207 (2007).
Diaz-Mitoma, F. et al. Transcriptional analysis of human peripheral blood mononuclear cells after influenza immunization. J. Clin. Virol. 31, 100–112 (2004).
Dhiman, N. et al. Immune activation at effector and gene expression levels after measles vaccination in healthy individuals: a pilot study. Hum. Immunol. 66, 1125–1136 (2005).
Wallace, J. C., Korth, M. J., Diamond, D. L., Proll, S. C. & Katze, M. G. Virology in the 21st century: finding function with functional genomics. Future Virol. 1, 47–53 (2006).
Diamond, D. L. et al. Hepatoproteomics: applying proteomic technologies to the study of liver function and disease. Hepatology 44, 299–308 (2006).
Maxwell, K. L. & Frappier, L. Viral proteomics. Microbiol. Mol. Biol. Rev. 71, 398–411 (2007).
Viswanathan, K. & Fruh, K. Viral proteomics: global evaluation of viruses and their interaction with the host. Expert Rev. Proteomics 4, 815–829 (2007).
Baas, T. et al. Integrated molecular signature of disease: analysis of influenza virus-infected macaques through functional genomics and proteomics. J. Virol. 80, 10813–10828 (2006).
Lim, L. P. et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 (2005).
Pfeffer, S. et al. Identification of microRNAs of the herpesvirus family. Nature Methods 2, 269–276 (2005).
Brass, A. L. et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319, 921–926 (2008).
Cherry, S. et al. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2, e102 (2006).
Ng, T. I. et al. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology 45, 1413–1421 (2007).
Li, J. & Burmeister, M. Genetical genomics: combining genetics with gene expression analysis. Hum. Mol. Genet. 14, R163–R169 (2005).
Bao, L. et al. An integrative genomics strategy for systematic characterization of genetic loci modulating phenotypes. Hum. Mol. Genet. 16, 1381–1390 (2007).
Drake, T. A., Schadt, E. E. & Lusis, A. J. Integrating genetic and gene expression data: application to cardiovascular and metabolic traits in mice. Mamm. Genome 17, 466–479 (2006).
Bystrykh, L. et al. Uncovering regulatory pathways that affect hematopoietic stem cell function using 'genetical genomics'. Nature Genet. 37, 225–232 (2005).
Gilbert, D. et al. Computational methodologies for modelling, analysis and simulation of signalling networks. Brief Bioinform. 7, 339–353 (2006).
Joyce, A. R. & Palsson, B. O. The model organism as a system: integrating 'omics' data sets. Nature Rev. Mol. Cell Biol. 7, 198–210 (2006).
Tan, S. L., Ganji, G., Paeper, B., Proll, S. & Katze, M. G. Systems biology and the host response to viral infection. Nature Biotechnol. 25, 1383–1389 (2007).
McKusick, V. A. & Ruddle, F. H. A new discipline, a new name, a new journal. Genomics 1, 1–2 (1987).
Ness, S. A. Microarray analysis: basic strategies for successful experiments. Mol. Biotechnol. 36, 205–219 (2007).
Ehrenreich, A. DNA microarray technology for the microbiologist: an overview. Appl. Microbiol. Biotechnol. 73, 255–273 (2006).
Bryant, P. A., Venter, D., Robins-Browne, R. & Curtis, N. Chips with everything: DNA microarrays in infectious diseases. Lancet Infect. Dis. 4, 100–111 (2004).
Braga-Neto, U. M. & Marques, E. T. Jr. From functional genomics to functional immunomics: new challenges, old problems, big rewards. PLoS Comput. Biol. 2, e81 (2006).
Neuman de Vegar, H. E. et al. Microarray profiling of antibody responses against simian-human immunodeficiency virus: postchallenge convergence of reactivities independent of host histocompatibility type and vaccine regimen. J. Virol. 77, 11125–11138 (2003).
Neuman de Vegar, H. E. & Robinson, W. H. Microarray profiling of antiviral antibodies for the development of diagnostics, vaccines, and therapeutics. Clin. Immunol. 111, 196–201 (2004).
Takahasi, K. et al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell 29, 428–440 (2008).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 11 June 2008 (doi:10.1038/nature07106).
Kato, H. et al. The length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 30 June 2008 (doi:10.1084/jem.20080091).
Acknowledgements
We thank B. Paeper and S. Proll for discussions and assistance with preparation of the original figures. Research in the authors' laboratory is supported by Public Health Service grants (R01AI022646, R01HL080621, R21AI017892, R24RR016354, P01AI052106, P01AI058113, P30DA015625 and P51RR000166) from the National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- IFN-stimulated genes
-
These genes contain interferon (IFN)-responsive promoters and are responsible for the antiviral, antiproliferative and immunomodulatory properties of IFN. Over 400 such genes have been identified by microarray analysis. Some, such as protein kinase R, ribonuclease L, Mx1 (myxovirus resistance 1) and ISG15 (IFN-stimulated protein of 15 kDa), have well documented antiviral activities, but the precise biological function of the majority of these genes is unknown.
- Chimeric SCID-Alb/uPA mouse model
-
A chimeric mouse model of severe combined immunodeficient (SCID) mice that contain a urokinase plasminogen activator transgene driven by an albumin promoter (Alb/uPA). These mice can be transplanted with human hepatocytes to generate chimeric mouse–human livers, providing the only small-animal infection model for hepatitis C virus infection.
- MicroRNAs
-
Single-stranded RNA molecules approximately 21–23 nucleotides in length that are thought to regulate the expression of other genes.
- Expression quantitative trait loci mapping
-
(eQTL mapping). Combination of QTL (regions of DNA that are closely linked to a phenotypic outcome) mapping and gene-expression analysis to study the genetic basis of gene expression and, by extension, biological regulation.
Rights and permissions
About this article
Cite this article
Katze, M., Fornek, J., Palermo, R. et al. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat Rev Immunol 8, 644–654 (2008). https://doi.org/10.1038/nri2377
Issue Date:
DOI: https://doi.org/10.1038/nri2377
This article is cited by
-
Molecular signatures in the progression of COVID-19 severity
Scientific Reports (2022)
-
β-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling
Acta Pharmacologica Sinica (2020)
-
Avian influenza virus directly infects human natural killer cells and inhibits cell activity
Virologica Sinica (2017)
-
Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection
Cellular & Molecular Immunology (2016)
-
Ubiquitin-specific Protease 15 Negatively Regulates Virus-induced Type I Interferon Signaling via Catalytically-dependent and -independent Mechanisms
Scientific Reports (2015)