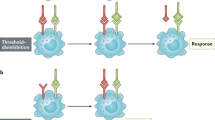
Key Points
-
The signal-regulatory proteins (SIRPs) can be classed as paired receptors, the most well known of which include the killer-cell immunoglobulin-like receptors, which are expressed by natural killer cells.
-
The SIRP family contains activating, inhibitory and non-signalling members, which have closely related extracellular regions but distinct cytoplasmic tails. They are expressed mainly by myeloid cells and are therefore thought to have a role in immune regulation.
-
Two types of ligand for the inhibitory member, SIRPα, have been identified: the widely expressed cell-surface protein CD47 and surfactant protein A.
-
A third SIRP-family member, SIRPγ, transmits neither activating nor inhibitory signals even though it binds CD47.
-
Like other paired receptors, the SIRPs show evidence of rapid evolution with considerable species differences and polymorphisms.
-
Factors such as ligand availability, binding affinity, protein mobility and expression levels are likely to affect their function in vivo.
Abstract
The immune system must be highly regulated to obtain optimal immune responses for the elimination of pathogens without causing undue side effects. This tight regulation involves complex interactions between membrane proteins on leukocytes. Members of the signal-regulatory protein (SIRP) family, which are expressed mainly by myeloid cells, provide one example of these regulatory membrane proteins. There are three SIRP-family genes that encode proteins that have similar extracellular regions but different signalling potentials, and are therefore known as 'paired receptors'. In this Review, we describe recent studies defining the ligands of the SIRP-family members, with particular emphasis on relating the molecular interactions of these proteins to their role in immune-cell regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bromley, S. K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Miller, M. J., Safrina, O., Parker, I. & Cahalan, M. D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 200, 847–856 (2004).
Kawakami, N. et al. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J. Exp. Med. 201, 1805–1814 (2005). The first identification of live trafficking of T cells at a site of inflammation using elegant imaging techniques.
Fujioka, Y. et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16, 6887–6899 (1996). The first identification of SIRPα at the primary sequence level and its association with a phosphatase.
Kharitonenkov, A. et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186 (1997).
Ohnishi, H., Kubota, M., Ohtake, A., Sato, K. & Sano, S. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J. Biol. Chem. 271, 25569–25574 (1996).
Veillette, A., Thibaudeau, E. & Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 273, 22719–22728 (1998).
Seiffert, M. et al. Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38− hematopoietic cells. Blood 97, 2741–2749 (2001). The first evidence that CD47 does not bind SIRPβ.
Ichigotani, Y. et al. Molecular cloning of a novel human gene (SIRP-B2) which encodes a new member of the SIRP/SHPS-1 protein family. J. Human Gen. 45, 378–382 (2000).
Dietrich, J., Cella, M., Seiffert, M., Buhring, H. J. & Colonna, M. Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 164, 9–12 (2000).
Tomasello, E. et al. Association of signal-regulatory proteins β with KARAP/DAP-12. Eur. J. Immunol. 30, 2147–2156 (2000).
Tomasello, E. & Vivier, E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur. J. Immunol. 35, 1670–1677 (2005).
Lanier, L. L. & Bakker, A. B. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today 21, 611–614 (2000).
Liu, Y. et al. SIRPβ1 is expressed as a disulfide linked homodimer in leukocytes and positively regulates neutrophil transepithelial migration. J. Biol. Chem. 280, 36132–36140 (2005). This paper shows that SIRPβ is a dimer, and the presence of intracellular pools of SIRPα.
Brooke, G., Holbrook, J. D., Brown, M. H. & Barclay, A. N. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 173, 2562–2570 (2004). Characterization of CD47 as a low-affinity ligand of SIRPγ.
Piccio, L. et al. Adhesion of human T cells to antigen-presenting cells through SIRPβ2–CD47 interaction costimulates T-cell proliferation. Blood 105, 2421–2427 (2005). Functional effects of SIRPγ in T-cell adhesion through CD47.
van Beek, E. M., Cochrane, F., Barclay, A. N. & van den Berg, T. K. Signal regulatory proteins in the immune system. J. Immunol. 175, 7781–7787 (2005).
Sano, S., Ohnishi, H. & Kubota, M. Gene structure of mouse BIT/SHPS-1. Biochem. J. 344, 667–675 (1999).
van den Berg, T. K., Yoder, J. A. & Litman, G. W. On the origins of adaptive immunity: innate immune receptors join the tale. Trends Immunol. 25, 11–16 (2004). Review of the function and origins of SIRPs.
Jiang, P., Lagenaur, C. F. & Narayanan, V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 274, 559–562 (1999). First identification of CD47 as a ligand for SIRPα expressed in the brain.
Seiffert, M. et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94, 3633–3643 (1999).
Vernon-Wilson, E. F. et al. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPα1. Eur. J. Immunol. 30, 2130–2137 (2000).
Latour, S. et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-α: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J. Immunol. 167, 2547–2554 (2001).
Manna, P. P. & Frazier, W. A. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J. Immunol. 170, 3544–3553 (2003).
Brown, E. J. & Frazier, W. A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130–135 (2001). Comprehensive review of CD47.
Gardai, S. J. et al. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115, 13–23 (2003). This paper shows that alveolar macrophages bind strongly to collectins through SIRPα.
Yamao, T. et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 277, 39833–39839 (2002).
Okazawa, H. et al. Negative regulation of phagocytosis in macrophages by the CD47–SHPS-1 system. J. Immunol. 174, 2004–2011 (2005).
Smith, R. E. et al. A novel MyD-1 (SIRP-1α) signaling pathway that inhibits LPS-induced TNFα production by monocytes. Blood 102, 2532–2540 (2003).
Liu, Y. et al. Signal regulatory protein (SIRPα), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 277, 10028–10036 (2002).
Inagaki, K. et al. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 19, 6721–6731 (2000).
Oshima, K., Ruhul Amin, A. R., Suzuki, A., Hamaguchi, M. & Matsuda, S. SHPS-1, a multifunctional transmembrane glycoprotein. FEBS Lett. 519, 1–7 (2002). Review on SIRPα with comprehensive analysis of functional data.
Timms, J. F. et al. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9, 927–930 (1999).
Alblas, J. et al. Signal regulatory protein α ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol. Cell. Biol. 25, 7181–7192 (2005).
Lindberg, F. P. et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274, 795–798 (1996).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Ishikawa-Sekigami, T. et al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 107, 341–348 (2006).
McVicar, D. W. et al. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 273, 32934–32942 (1998).
Hayashi, A. et al. Positive regulation of phagocytosis by SIRPβ and its signaling mechanism in macrophages. J. Biol. Chem. 279, 29450–29460 (2004).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Vales-Gomez, M., Reyburn, H. T., Erskine, R. A. & Strominger, J. Differential binding to HLA-C of p50-activating and p58-inhibitory natural killer cell receptors. Proc. Natl Acad. Sci. USA 95, 14326–14331 (1998).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Wild, M. K. et al. Dependence of T cell antigen recognition on the dimensions of an accessory receptor–ligand complex. J. Exp. Med. 190, 31–41 (1999).
Choudhuri, K., Wiseman, D., Brown, M. H., Gould, K. & van der Merwe, P. A. T-cell receptor triggering is critically dependent on the dimensions of its peptide–MHC ligand. Nature 436, 578–582 (2005).
Barclay, A. N. et al. Leucocyte Antigens Factsbook 2nd edn (Academic Press, London, 1997). Reviews the concepts about domain organization, size and affinity of interactions of leukocyte membrane proteins.
Wright, G. J. et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242 (2000).
Ohnishi, H. et al. Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J. Neurosci. 25, 2702–2711 (2005).
Mi, Z. P. et al. Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J. Comp. Neurol. 416, 335–344 (2000).
Rebres, R. A., Vaz, L. E., Green, J. M. & Brown, E. J. Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane-spanning domains. J. Biol. Chem. 276, 34607–34616 (2001).
Jimenez, D., Roda-Navarro, P., Springer, T. A. & Casasnovas, J. M. Contribution of N-linked glycans to the conformation and function of intercellular adhesion molecules (ICAMs). J. Biol. Chem. 280, 5854–5861 (2005).
Ogura, T. et al. Resistance of B16 melanoma cells to CD47-induced negative regulation of motility as a result of aberrant N-glycosylation of SHPS-1. J. Biol. Chem. 279, 13711–13720 (2004).
LeVine, A. M. et al. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165, 3934–3940 (2000).
van der Merwe, P. A. & Davis, S. J. Molecular interactions mediating T cell ntigen recognition. Annu. Rev. Immunol. 21, 659–684 (2003).
van der Merwe, P. A. & Barclay, A. N. Analysis of cell-adhesion molecule interactions using surface plasmon resonance. Curr. Opin. Immunol. 8, 257–261 (1996).
Foster-Cuevas, M., Wright, G. J., Puklavec, M. J. & Barclay, A. N. Human herpesvirus-8 K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J. Virol. 78, 7667–7676 (2004). This paper shows that a viral homologue of CD200 can downmodulate the activity of activated macrophages through an inhibitory receptor.
Collins, A. V. et al. The interaction properties of costimulatory molecules revisited. Immunity 17, 201–210 (2002).
Dustin, M. L. et al. Low affinity interaction of human or rat T cell adhesion molecule CD2 with its ligand aligns adhering membranes to achieve high physiological affinity. J. Biol. Chem. 272, 30889–30898 (1997).
Douglass, A. D. & Vale, R. D. Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950 (2005).
Koch, C. et al. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 11, 777–786 (1999).
Smith, H. R. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl Acad. Sci. USA 99, 8826–8831 (2002). Key finding that the presence of activating and inhibitory Ly49 receptors can dictate resistance versus susceptibility to virus infection.
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002). This paper shows that the interaction of activating and inhibitory Ly49 proteins with a viral protein is the basis of strain susceptibility to MCMV.
Smith, K. M., Wu, J., Bakker, A. B., Phillips, J. H. & Lanier, L. L. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J. Immunol. 161, 7–10 (1998).
Bubic, I. et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J. Virol. 78, 7536–7544 (2004).
Sano, S., Ohnishi, H., Omori, A., Hasegawa, J. & Kubota, M. BIT, an immune antigen receptor-like molecule in the brain. FEBS Lett. 411, 327–334 (1997).
Koshimizu, H. et al. Expression of CD47/integrin-associated protein induces death of cultured cerebral cortical neurons. J. Neurochem. 82, 249–257 (2002).
Miyashita, M. et al. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol. Biol. Cell 15, 3950–3963 (2004).
Williams, A. F. & Barclay, A. N. The immunoglobulin superfamily — domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405 (1988).
Barclay, A. N., Wright, G. J., Brooke, G. & Brown, M. H. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 23, 285–290 (2002).
Wright, G. J. et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 171, 3034–3046 (2003).
Voehringer, D., Rosen, D. B., Lanier, L. L. & Locksley, R. M. CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. J. Biol. Chem. 279, 54117–54123 (2004).
Cameron, C. M., Barrett, J. W., Mann, M., Lucas, A. & McFadden, G. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology 337, 55–67 (2005).
Cameron, C. M., Barrett, J. W., Liu, L., Lucas, A. R. & McFadden, G. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J. Virol. 79, 6052–6067 (2005).
van den Berg, T. K. et al. A nomenclature for signal regulatory protein family members. J. Immunol. 175, 7788–7789 (2005).
Shiratori, I., Ogasawara, K., Saito, T., Lanier, L. L. & Arase, H. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J. Exp. Med. 199, 525–533 (2004).
Allen, R. L., Raine, T., Haude, A., Trowsdale, J. & Wilson, M. J. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J. Immunol. 167, 5543–5547 (2001).
Borges, L. & Cosman, D. LIRs/ILTs/MIRs, inhibitory and stimulatory Ig-superfamily receptors expressed in myeloid and lymphoid cells. Cytokine Growth Factor Rev. 11, 209–217 (2000).
Aguilar, H. et al. Molecular characterization of a novel immune receptor restricted to the monocytic lineage. J. Immunol. 173, 6703–6711 (2004).
Chung, D. H. et al. CMRF-35-like molecule-1, a novel mouse myeloid receptor, can inhibit osteoclast formation. J. Immunol. 171, 6541–6548 (2003).
Clark, G. J., Green, B. J. & Hart, D. N. The CMRF-35H gene structure predicts for an independently expressed member of an ITIM/ITAM pair of molecules localized to human chromosome 17. Tissue Antigens 55, 101–109 (2000).
Bates, E. E. et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 163, 1973–1983 (1999).
Kanazawa, N., Tashiro, K., Inaba, K. & Miyachi, Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor γ chain. J. Biol. Chem. 278, 32645–32652 (2003).
Kanazawa, N., Tashiro, K. & Miyachi, Y. Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2. Immunobiology 209, 179–190 (2004). Extensive review of paired receptors on dendritic cells.
Hatherley, D., Cherwinski, H. M., Moshref, M. & Barclay, A. N. Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. J. Immunol. 175, 2469–2474 (2005).
Dietrich, J., Nakajima, H. & Colonna, M. Human inhibitory and activating Ig-like receptors which modulate the function of myeloid cells. Microbes Infect. 2, 323–329 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Immunological synapse
-
A region that can form between two cells of the immune system that are in close contact, so named because of its similarities to the synapses that occur in the nervous system. The immunological synapse originally referred to the interaction between a T cell and an antigen-presenting cell, but it is now known to form between other types of immune cell, for example, between dendritic cells and natural killer cells. It involves adhesion molecules, as well as antigen receptors and cytokine receptors.
- Immunoreceptor tyrosine-based inhibitory motif
-
(ITIM). A short peptide motif that contains tyrosine residues and is found in the cytoplasmic tails of several inhibitory receptors. The prototype six-amino-acid ITIM sequence is (I/V/L/S)XYXX(L/V), with X denoting any amino acid. Ligand-induced clustering of these inhibitory receptors results in tyrosine phosphorylation, often by SRC-family protein tyrosine kinases, and this provides a docking site for the recruitment of cytoplasmic phosphatases that have an SRC homology 2 (SH2) domain.
- Immunoglobulin superfamily domain
-
(IgSF domain). This domain type shows sequence similarity to domains present in immunoglobulins and is the most common domain type of leukocyte surface proteins.
- Domain
-
Region of a protein that forms a discrete fold. The extracellular regions of leukocyte surface proteins contain several common domain types, such as immunoglobulin superfamily and C-type lectin domains.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A short peptide motif containing tyrosine residues that is found in the cytoplasmic tails of several signalling molecules. The amino-acid sequence of an ITAM is (D/E)XXYXX(L/I)X6–8YXX(L/I), with X denoting any amino acid. It is tyrosine phosphorylated after engagement of the ligand-binding subunits, which triggers a cascade of intracellular events that results in cellular activation.
- Polymorphic
-
In population genetics, this term refers to genes that, in a population, have many alleles.
- Collectin
-
C-type lectins that have a collagen-like domain are known as collectins. One group of collectins, the secreted lectins, consists of mannose-binding lectin (MBL), bovine conglutinin and collectin-43 (CL43) in blood and the two mucosal-associated proteins surfactant protein A (SP-A) and SP-D. The other group of collectins consists of the newly discovered, non-secreted-type collectin liver 1 (CLL1) and membrane-type collectin placenta 1 (CLP1).
- Erythropoiesis
-
The differentiation process leading from a committed erythroid precursor to the production of mature red blood cells (erythrocytes).
- Thrombocytopaenia
-
A reduced number of circulating platelets, owing to either the failure of production from bone-marrow megakaryocytes or increased clearance from the circulation, predominantly in the spleen.
Rights and permissions
About this article
Cite this article
Barclay, A., Brown, M. The SIRP family of receptors and immune regulation. Nat Rev Immunol 6, 457–464 (2006). https://doi.org/10.1038/nri1859
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1859
This article is cited by
-
Discovery of a novel small molecule as CD47/SIRPα and PD-1/PD-L1 dual inhibitor for cancer immunotherapy
Cell Communication and Signaling (2024)
-
Surfactant protein D prevents mucin overproduction in airway goblet cells via SIRPα
Scientific Reports (2024)
-
The DNA damage response pathway regulates the expression of the immune checkpoint CD47
Communications Biology (2023)
-
Innate Immune Responses in Transplant Immunity
Current Transplantation Reports (2023)
-
Targeting CD47/SIRPα as a therapeutic strategy, where we are and where we are headed
Biomarker Research (2022)