Key Points
-
Viruses elicit a broad spectrum of antibody responses. By focusing on two experimental virus infections — the non-cytopathic, persistency-prone lymphocytic choriomeningitis virus (LCMV) and the acutely cytopathic, rabies-like vesicular stomatitis virus (VSV) in mice — we outline two fundamentally different types of antiviral antibody response. These results are discussed in the context of other viral infections, such as influenza virus and HIV.
-
The immunogenicity of viral surface proteins is influenced by various factors, including accessibility, glycosylation, repetitiveness and organization. The availability of B cells that encode immunoglobulin that is specific for these different viral epitopes also has a great impact on their immunogenicity.
-
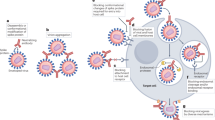
Acutely cytopathic virus infections, such as VSV, must be controlled rapidly before the virus can spread to vital organs and kill the host. Therefore, most cytopathic viruses support the rapid generation of neutralizing antibodies.
-
Poorly or non-cytopathic viruses, such as LCMV, tend to persist and actively interfere with the generation of neutralizing antibodies. These viruses are frequently transmitted vertically from a virus-carrier mother to her immuno-incompetent offspring.
-
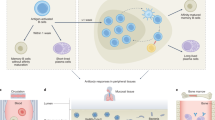
Spleen and bone marrow have a limited capacity to accommodate antibody-producing plasma cells. However, IgM has a short serum half-life, and protective IgM titres require many plasma cells to be maintained. The longer serum half-life of IgG isotype class-switched antibodies allows protective titres to be maintained by fewer plasma cells. In addition, IgG antibodies can be transferred to the infant for protection during the early period of immunoincompentence.
-
There is evidence that germline-encoded antibodies can provide early and sufficient protection against cytopathic viruses, without undergoing somatic hypermutation (SHM). By contrast, extensive SHM and affinity maturation are required for the formation of protective humoral responses against poorly cytopathic viruses that have a tendency to persist. In these cases, the germline repertoire does not normally provide antibodies with sufficient affinity to be immediately protective.
-
Viruses tend to escape neutralizing antibody responses. In general, rapidly transmitting, acutely cytopathic, and therefore lethal, viruses have to evade herd immunity and therefore frequently exist in closely related but immunologically distinct varieties (serotypes). By contrast, poorly cytopathic viruses evade the immune response of the individual host by diversifying into numerous quasi-species, with individual neutralizing specificities within a single infected individual.
Abstract
Viruses elicit a diverse spectrum of antiviral antibody responses. In this review, we discuss two widely used experimental model systems for viral infections — non-cytopathic lymphocytic choriomeningitis virus (LCMV) and acutely cytopathic vesicular stomatitis virus (VSV) — to analyse two fundamentally different types of antiviral antibody response. The basic principles found in these model infections are discussed in the context of other viral infections, and with regard to protective neutralizing versus non-protective enzyme-linked immunosorbent assay (ELISA)-detected antibody responses. Issues of antibody specificity, affinity and avidity, maturation and escape are discussed in the context of co-evolution of the host and viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zuniga, M. C. A pox on thee! Manipulation of the host immune system by myxoma virus and implications for viral–host co-adaptation. Virus Res. 88, 17–33 (2002).
Traub, E. The epidemiology of lymphocytic choriomeningitis in white mice. J. Exp. Med. 64, 183–200 (1936).
Jonas, M. M. Children with hepatitis C. Hepatology 36, S173–S178 (2002).
Yeoh, E. K. Hepatitis B virus infection in children. Vaccine 8, S29–S30 (1990).
Whitley, R. J., Kimberlin, D. W. & Roizman, B. Herpes simplex viruses. Clin. Infect. Dis. 26, 541–553 (1998).
Stubenrauch, F. & Laimins, L. A. Human papillomavirus life cycle: active and latent phases. Semin. Cancer Biol. 9, 379–386 (1999).
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994). This study shows that without interferons, the adaptive immune system is completely helpless in its defense against viruses.
Buchmeier, M. J. & Oldstone, M. B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J. Immunol. 120, 1297–1304 (1978).
Maruyama, T. et al. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 73, 6024–6030 (1999).
Roost, H. P. et al. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc. Natl Acad. Sci. USA 92, 1257–1261 (1995).
Bachmann, M. F. et al. The role of antibody concentration and avidity in antiviral protection. Science 276, 2024–2027 (1997). This study indicates that somatic hypermutation has little influence on in vivo protection against VSV.
Saphire, E. O. et al. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science 293, 1155–1159 (2001).
Smith, T. J., Chase, E. S., Schmidt, T. J., Olson, N. H. & Baker, T. S. Neutralizing antibody to human rhinovirus 14 penetrates the receptor-binding canyon. Nature 383, 350–354 (1996).
Bizebard, T. et al. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature 376, 92–94 (1995).
Roben, P. et al. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68, 4821–4828 (1994).
Fleury, D. et al. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nature Struct. Biol. 6, 530–534 (1999).
Emini, E. A., Kao, S. Y., Lewis, A. J., Crainic, R. & Wimmer, E. Functional basis of poliovirus neutralization determined with monospecific neutralizing antibodies. J. Virol. 46, 466–474 (1983).
Schibli, D. J. & Weissenhorn, W. Class I and class II viral fusion protein structures reveal similar principles in membrane fusion. Mol. Membr. Biol. 21, 361–371 (2004).
Skehel, J. J. & Wiley, D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569 (2000). A review that summarizes the structural and functional data available for influenza haemagglutinin.
Chan, D. C. & Kim, P. S. HIV entry and its inhibition. Cell 93, 681–684 (1998).
Outlaw, M. C. & Dimmock, N. J. IgG neutralization of type A influenza viruses and the inhibition of the endosomal fusion stage of the infectious pathway in BHK cells. Virology 195, 413–421 (1993).
Battegay, M., Kyburz, D., Hengartner, H. & Zinkernagel, R. M. Enhancement of disease by neutralizing antiviral antibodies in the absence of primed antiviral cytotoxic T cells. Eur. J. Immunol. 23, 3236–3241 (1993).
Parren, P. W., Burton, D. R. & Sattentau, Q. J. HIV-1 antibody — debris or virion? Nature Med. 3, 366–367 (1997).
Leung, D. T. et al. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J. Infect. Dis. 190, 379–386 (2004).
Sakurai, H. et al. Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines. J. Virol. 73, 2956–2962 (1999).
Sattentau, Q. J. & Moore, J. P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182, 185–196 (1995).
Lefrancois, L. & Lyles, D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology 121, 168–174 (1982).
Charan, S., Hengartner, H. & Zinkernagel, R. M. Antibodies against the two serotypes of vesicular stomatitis virus measured by enzyme-linked immunosorbent assay: immunodominance of serotype-specific determinants and induction of asymmetrically cross-reactive antibodies. J. Virol. 61, 2509–2514 (1987). Prototypical description of the antibody response in mice that are primed with one serotype and challenged with a different serotype of the same virus.
Murphy, B. R. et al. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J. Clin. Microbiol. 13, 554–560 (1981).
Giessauf, A., Letschka, T., Walder, G., Dierich, M. P. & Wurzner, R. A synthetic peptide ELISA for the screening of rubella virus neutralizing antibodies in order to ascertain immunity. J. Immunol. Methods 287, 1–11 (2004).
Usonis, V., Bakasenas, V. & Denis, M. Neutralization activity and persistence of antibodies induced in response to vaccination with a novel mumps strain, RIT 4385. Infection 29, 159–162 (2001).
Battegay, M. et al. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 151, 5408–5415 (1993); erratum 152, 1635 (1994).
Cafruny, W. A. et al. Antibody response of mice to lactate dehydrogenase-elevating virus during infection and immunization with inactivated virus. Virus Res. 5, 357–375 (1986).
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003).
Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl Acad. Sci. USA 100, 4144–4149 (2003). References 34 and 35 describe the 'race' between the generation of HIV-neutralizing antibodies and the emergence of antibody-escape viral variants.
Aasa-Chapman, M. M. et al. Development of the antibody response in acute HIV-1 infection. AIDS 18, 371–381 (2004).
Lefrancois, L. & Lyles, D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 121, 157–167 (1982).
Rossmann, M. G. et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317, 145–153 (1985).
Wilson, I. A., Skehel, J. J. & Wiley, D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289, 366–373 (1981).
Pantophlet, R., Wilson, I. A. & Burton, D. R. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J. Virol. 77, 5889–5901 (2003).
Wright, K. E., Salvato, M. S. & Buchmeier, M. J. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology 171, 417–426 (1989).
Dintzis, H. M., Dintzis, R. Z. & Vogelstein, B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc. Natl Acad. Sci. USA 73, 3671–3675 (1976).
Feldmann, M., Howard, J. G. & Desaymard, C. Role of antigen structure in the discrimination between tolerance and immunity by B cells. Transplant. Rev. 23, 78–97 (1975).
Pinschewer, D. D. et al. Kinetics of protective antibodies are determined by the viral surface antigen. J. Clin. Invest. 114, 988–993 (2004).
Hangartner, L. et al. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc. Natl Acad. Sci. USA 100, 12883–12888 (2003).
Haury, M. et al. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur. J. Immunol. 27, 1557–1563 (1997).
Baumgarth, N., Herman, O. C., Jager, G. C., Brown, L. & Herzenberg, L. A. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl Acad. Sci. USA 96, 2250–2255 (1999).
Ditzel, H. J., Itoh, K. & Burton, D. R. Determinants of polyreactivity in a large panel of recombinant human antibodies from HIV-1 infection. J. Immunol. 157, 739–749 (1996).
Torán, J. L. et al. Molecular analysis of HIV-1 gp120 antibody response using isotype IgM and IgG phage display libraries from a long-term non-progressor HIV-1-infected individual. Eur. J. Immunol. 29, 2666–2675 (1999). This study describes the generation of increased antibody specificity by somatic hypermutation.
Ochsenbein, A. F. & Zinkernagel, R. M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21, 624–630 (2000). A review summarizing the importance of natural antibodies in the abatement of pathogens.
Ochsenbein, A. F. et al. Correlation of T cell independence of antibody responses with antigen dose reaching secondary lymphoid organs: implications for splenectomized patients and vaccine design. J. Immunol. 164, 6296–6302 (2000).
Shibuya, A. et al. Fc a/m receptor mediates endocytosis of IgM-coated microbes. Nature Immunol. 1, 441–446 (2000).
Kopf, M., Abel, B., Gallimore, A., Carroll, M. & Bachmann, M. F. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nature Med. 8, 373–378 (2002).
van Noesel, C. J., Lankester, A. C. & van Lier, R. A. Dual antigen recognition by B cells. Immunol. Today 14, 8–11 (1993).
Ochsenbein, A. F. et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286, 2156–2159 (1999).
Martinez, I., Barrera, J. C., Rodriguez, L. L. & Wertz, G. W. Recombinant vesicular stomatitis (Indiana) virus expressing New Jersey and Indiana glycoproteins induces neutralizing antibodies to each serotype in swine, a natural host. Vaccine 22, 4035–4043 (2004).
Baumgarth, N. et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192, 271–280 (2000).
Kopf, M., Brombacher, F. & Bachmann, M. F. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur. J. Immunol. 32, 2229–2236 (2002).
Deshpande, S. P., Kumaraguru, U. & Rouse, B. T. Dual role of B cells in mediating innate and acquired immunity to herpes simplex virus infections. Cell. Immunol. 202, 79–87 (2000).
Seiler, P. et al. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 27, 2626–2633 (1997).
Oehen, S. et al. Marginal zone macrophages and immune responses against viruses. J. Immunol. 169, 1453–1458 (2002).
Hidalgo, S., Garcia Erro, M., Cisterna, D. & Freire, M. C. Paralytic poliomyelitis caused by a vaccine-derived polio virus in an antibody-deficient Argentinean child. Pediatr. Infect. Dis. J. 22, 570–572 (2003).
Thomsen, A. R. et al. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 9, 1757–1766 (1997).
Bründler, M. A. et al. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26, 2257–2262 (1996).
Diamond, M. S. et al. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198, 1853–1862 (2003).
Lutz, C. et al. IgD can largely substitute for loss of IgM function in B cells. Nature 393, 797–801 (1998).
Burns, W., Billups, L. C. & Notkins, A. L. Thymus dependence of viral antigens. Nature 256, 654–656 (1975).
Bachmann, M. F., Ecabert, B. & Kopf, M. Influenza virus: a novel method to assess viral and neutralizing antibody titers in vitro. J. Immunol. Methods 225, 105–111 (1999).
Fehr, T. et al. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nature Med. 4, 945–948 (1998).
Szomolanyi-Tsuda, E. & Welsh, R. M. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 10, 431–435 (1998).
Alimonti, J. B., Ball, T. B. & Fowke, K. R. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84, 1649–1661 (2003).
Racz, P. et al. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog. Allergy 37, 81–181 (1986).
Frankel, S. S. et al. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272, 115–117 (1996).
de Roda Husman, A. M., van Rij, R. P., Blaak, H., Broersen, S. & Schuitemaker, H. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180, 1106–1115 (1999).
Hunziker, L. et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nature Immunol. 4, 343–349 (2003).
Recher, M. et al. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nature Immunol. 5, 934–942 (2004).
Borrow, P., Evans, C. F. & Oldstone, M. B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69, 1059–1070 (1995).
Odermatt, B., Eppler, M., Leist, T. P., Hengartner, H. & Zinkernagel, R. M. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl Acad. Sci. USA 88, 8252–8256 (1991).
Roost, H. et al. An acquired immune suppression in mice caused by infection with lymphocytic choriomeningitis virus. Eur. J. Immunol. 18, 511–518 (1988).
Bachmann, M. F., Odermatt, B., Hengartner, H. & Zinkernagel, R. M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 183, 2259–2269 (1996).
Llorente, M. et al. Natural human antibodies retrieved by phage display libraries from healthy donors: polyreactivity and recognition of human immunodeficiency virus type 1 gp120 epitopes. Scand. J. Immunol. 50, 270–279 (1999).
Harindranath, N., Ikematsu, H., Notkins, A. L. & Casali, P. Structure of the VH and VL segments of polyreactive and monoreactive human natural antibodies to HIV-1 and Escherichia coli β-galactosidase. Int. Immunol. 5, 1523–1533 (1993).
Berberian, L., Valles-Ayoub, Y., Sun, N., Martinez-Maza, O. & Braun, J. A VH clonal deficit in human immunodeficiency virus-positive individuals reflects a B-cell maturational arrest. Blood 78, 175–179 (1991).
Neshat, M. N., Goodglick, L., Lim, K. & Braun, J. Mapping the B cell superantigen binding site for HIV-1 gp120 on a V(H)3 Ig. Int. Immunol. 12, 305–312 (2000).
De Milito, A. et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103, 2180–2186 (2004).
Dion, M. L. et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21, 757–768 (2004).
Tenner-Racz, K. Human immunodeficiency virus associated changes in germinal centers of lymph nodes and relevance to impaired B-cell function. Lymphology 21, 36–43 (1988).
Manz, R. A. & Radbruch, A. Plasma cells for a lifetime? Eur. J. Immunol. 32, 923–927 (2002).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Bachmann, M. F., Kundig, T. M., Kalberer, C. P., Hengartner, H. & Zinkernagel, R. M. How many specific B cells are needed to protect against a virus? J. Immunol. 152, 4235–4241 (1994).
Vieira, P. & Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18, 313–316 (1988).
Andersen, C., Jensen, T., Nansen, A., Marker, O. & Thomsen, A. R. CD4(+) T cell-mediated protection against a lethal outcome of systemic infection with vesicular stomatitis virus requires CD40 ligand expression, but not IFN-γ or IL-4. Int. Immunol. 11, 2035–2042 (1999).
Planz, O. et al. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc. Natl Acad. Sci. USA 94, 6874–6879 (1997).
Coutelier, J. P., van der Logt, J. T., Heessen, F. W., Warnier, G. & Van Snick, J. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165, 64–69 (1987).
Sangster, M. Y. et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J. Immunol. 164, 1820–1828 (2000).
Snapper, C. M. & Paul, W. E. Interferon-γ Ig and B cell stimulatory factor-1 reciprocally regulate isotype production. Science 236, 944–947 (1987).
Maloy, K. J. et al. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 191, 2159–2170 (2000).
Baldridge, J. R. & Buchmeier, M. J. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 66, 4252–4257 (1992).
Thomsen, A. R., Volkert, M. & Marker, O. Different isotype profiles of virus-specific antibodies in acute and persistent lymphocytic choriomeningitis virus infection in mice. Immunology 55, 213–223 (1985).
Wilson, J. A. et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287, 1664–1666 (2000).
Markine-Goriaynoff, D. & Coutelier, J. P. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. J. Virol. 76, 432–435 (2002).
Huber, V. C., Lynch, J. M., Bucher, D. J., Le, J. & Metzger, D. W. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166, 7381–7388 (2001).
Fleming, J. O., Shubin, R. A., Sussman, M. A., Casteel, N. & Stohlman, S. A. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology 168, 162–167 (1989).
Kumel, G., Kaerner, H. C., Levine, M., Schroder, C. H. & Glorioso, J. C. Passive immune protection by herpes simplex virus-specific monoclonal antibodies and monoclonal antibody-resistant mutants altered in pathogenicity. J. Virol. 56, 930–937 (1985).
Palladino, G., Mozdzanowska, K., Washko, G. & Gerhard, W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 69, 2075–2081 (1995).
Harada, Y., Muramatsu, M., Shibata, T., Honjo, T. & Kuroda, K. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J. Exp. Med. 197, 1779–1785 (2003).
Seiler, P. et al. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J. Exp. Med. 187, 649–654 (1998). A comparison of the requirements for sterile protection against a cytopathic and a non-cytopathic virus.
Graham, B. S., Bunton, L. A., Wright, P. F. & Karzon, D. T. Reinfection of mice with respiratory syncytial virus. J. Med. Virol. 34, 7–13 (1991).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Plotkin, S. A. Immunologic correlates of protection induced by vaccination. Pediatr. Infect. Dis. J. 20, 63–75 (2001).
Zinkernagel, R. M. On natural and artificial vaccinations. Annu. Rev. Immunol. 21, 515–546 (2003).
Shibata, R. et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature Med. 5, 204–210 (1999).
Parren, P. W. et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75, 8340–8347 (2001).
Alberti, A. et al. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet 331, 1421–1424 (1988).
Neurath, A. R., Seto, B. & Strick, N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 7, 234–236 (1989).
Jung, M. C. & Pape, G. R. Immunology of hepatitis B infection. Lancet Infect. Dis. 2, 43–50 (2002).
Burton, D. R. Antibodies, viruses and vaccines. Nature Rev. Immunol. 2, 706–713 (2002).
Griffiths, G. M., Berek, C., Kaartinen, M. & Milstein, C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature 312, 271–275 (1984).
Weiss, U., Zoebelein, R. & Rajewsky, K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur. J. Immunol. 22, 511–517 (1992).
Goldbaum, F. A. et al. Lack of significant differences in association rates and affinities of antibodies from short-term and long-term responses to hen egg lysozyme. J. Immunol. 162, 6040–6045 (1999).
Kalinke, U. et al. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity 5, 639–652 (1996). An analysis of monoclonal antibodies that were generated at various time points following infection, which shows that maturation of the antibody response against VSV is due, in part, to somatic hypermutation, but largely to the use of alternative V-region families during the late antibody response compared with the early response.
Clarke, S. H. et al. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. J. Immunol. 144, 2795–2801 (1990).
Oppezzo, P. et al. Somatic mutations can lead to a loss of superantigenic and polyreactive binding. Eur. J. Immunol. 34, 1423–1432 (2004).
Hilleman, M. R. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 20, 3068–3087 (2002).
de St Groth, F. & Webster, R. G. Disquisitions of original antigenic sin. I. Evidence in man. J. Exp. Med. 124, 331–345 (1966).
Ciurea, A. et al. Viral persistence in vivo through selection of neutralizing antibody- escape variants. Proc. Natl Acad. Sci. USA 97, 2749–2754 (2000).
Hunziker, L., Ciurea, A., Recher, M., Hengartner, H. & Zinkernagel, R. M. Public versus personal serotypes of a viral quasispecies. Proc. Natl Acad. Sci. USA 100, 6015–6020 (2003).
Seiler, P. et al. In vivo selection of neutralization-resistant virus variants but no evidence of B cell tolerance in lymphocytic choriomeningitis virus carrier mice expressing a transgenic virus-neutralizing antibody. J. Immunol. 162, 4536–4541 (1999).
Seiler, P. et al. Additive effect of neutralizing antibody and antiviral drug treatment in preventing virus escape and persistence. J. Virol. 74, 5896–5901 (2000).
Derdeyn, C. A. et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303, 2019–2022 (2004).
Moskophidis, D., Cobbold, S. P., Waldmann, H. & Lehmann-Grube, F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J. Virol. 61, 1867–1874 (1987).
Zinkernagel, R. M., Leist, T., Hengartner, H. & Althage, A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J. Exp. Med. 162, 2125–2141 (1985).
Cerny, A., Sutter, S., Bazin, H., Hengartner, H. & Zinkernagel, R. M. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell- deprived mice. J. Virol. 62, 1803–1807 (1988).
Bruns, M., Cihak, J., Muller, G. & Lehmann-Grube, F. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology 130, 247–251 (1983).
Buchmeier, M. J., Welsh, R. M., Dutko, F. J. & Oldstone, M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 30, 275–331 (1980).
Ciurea, A., Hunziker, L., Zinkernagel, R. M. & Hengartner, H. Viral escape from the neutralizing antibody response: the lymphocytic choriomeningitis virus model. Immunogenetics 53, 185–189 (2001).
Ochsenbein, A. F. et al. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl Acad. Sci. USA 97, 13263–13268 (2000).
Leist, T. P., Cobbold, S. P., Waldmann, H., Aguet, M. & Zinkernagel, R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 138, 2278–2281 (1987).
Acknowledgements
We are grateful to B. Eschli for sharing unpublished data regarding the LCMV-glycoprotein-specific antibody response in wild-type mice and for helpful discussions during the preparation of the manuscript. We also thank K. McCoy and N. Harris for critical reading of the manuscript, together with A. Trkola, M. Recher, H.C. Probst, K. Fink and R. Zellweger for discussions and reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Latent
-
Reversible dormant state of viruses in infected cells with minimal production of viral proteins and absence of progeny virus production.
- Complementarity-determining region
-
(CDR). The most variable parts of immunoglobulin molecules and T-cell receptors. These regions form loops that make contact with specific ligands. There are three such regions (CDR1, CDR2 and CDR3) contained in each V domain, with CDR3 arising from V(D)J rearrangement, and therefore being the most variable CDR.
- T-cell-independent antigen/T-cell-dependent antigen
-
Antigens that require specific T-cell help for eliciting antibody responses are designated as T-cell-dependent antigens. By contrast, T-cell-independent antigens elicit IgM antibody responses in the absence of specific T-cell help.
- Epitope
-
The part of an antigen that is directly recognized by antibodies or T-cell receptors.
- ELISA
-
(Enzyme-linked immunosorbent assay). This assay can be used to detect antibodies that bind to an antigen, which is immobilized on a plastic surface. Samples to be tested are incubated on the coated plastic plates to allow binding of the contained antibodies to the coated antigen. Bound antibodies are then detected through an anti-immunoglobulin antibody coupled to an enzyme, which can catalyse a colour reaction.
- Neutralization assay
-
An in vitro assay used to detect direct antiviral activities of antibodies. Constant amounts of infectious virus are incubated with serially diluted antibodies and neutralizing titres are defined as the dilution that reduces infectivity in cell cultures by at least 50%.
- Hapten
-
A small molecule, or part of a molecule, that can elicit antibody responses when it is chemically linked to a carrier, but that is not immunogenic by itself. B-cell responses against haptens require priming of T-helper cells that are specific for the carrier, unless they are repetitively linked to a rigid carrier at a distance of 5–10 nm.
- B-cell superantigen
-
Antigen that is able to activate B cells by signalling through the B-cell receptor, irrespective of the specificity of its immunoglobulin component. B-cell superantigens usually bind to areas of immunoglobulin molecules that are not involved in normal antigen recognition.
- On rate/off rate
-
Binding of a ligand to its receptor (for example, antigen to antibody) is determined by the on-rate, describing the kinetics by which the ligand is bound by the receptor, and the off-rate, describing the kinetics by which bound ligand is released from the receptor. In the equilibrium, the amount of complexed ligand is determined by the ratio between the on- and the off-rate.
- Somatic hypermutation
-
(SHM). The process by which antigen-activated B cells in germinal centres mutate the rearranged immunoglobulin genes. The B cells are subsequently selected for those that express the 'best' mutations on the basis of the ability of the surface immunoglobulin to bind antigen.
- Long-term non-progressor
-
(LTNP). There is no universally adopted definition of LTNP, and often LTNP is used interchangeably with HIV controller, because control of viremia is strongly predictive of LNTP. So, LTNP refers to untreated HIV-infected individuals who are infected for more than 10 years, whose CD4+ T-cell counts and viral load are stable over time and who are free of disease.
- Heterologously primed
-
Hosts that are immune against a different virus or serotype than that used for a secondary infection.
- Antigenic drift
-
Continuous mutation of surface antigens used by influenza viruses to gain resistance against antibody responses that are elicited by previous, less mutated variants.
- Antigenic shift
-
Emergence of influenza viruses that use surface antigens that differ fundamentally from those of currently circulating strains. The acquisition of relevant genome segments of avian origin by human influenza viruses is thought to be mainly responsible for these antigenic shifts.
- Original antigenic sin
-
A term used to describe the phenomenon in which infections with drifted variants of influenza viruses boost memory responses against the virus that has been encountered first.
Rights and permissions
About this article
Cite this article
Hangartner, L., Zinkernagel, R. & Hengartner, H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol 6, 231–243 (2006). https://doi.org/10.1038/nri1783
Issue Date:
DOI: https://doi.org/10.1038/nri1783
This article is cited by
-
Unmodified rabies mRNA vaccine elicits high cross-neutralizing antibody titers and diverse B cell memory responses
Nature Communications (2023)
-
Targeting BMI-1 in B cells restores effective humoral immune responses and controls chronic viral infection
Nature Immunology (2022)
-
Protective and pathogenic role of humoral responses in COVID-19
Journal of Microbiology (2022)
-
Case Series: Convalescent Plasma Therapy for Patients with COVID-19 and Primary Antibody Deficiency
Journal of Clinical Immunology (2022)
-
Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT – IRCCS “Fondazione Pascale” Cancer Center (Naples, Italy)
Infectious Agents and Cancer (2022)