Key Points
-
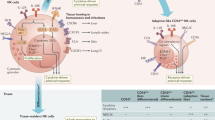
Natural killer (NK) cells are a subset of lymphoid cells that function as important mediators of the innate immune defence mechanisms against viruses and tumour cells. As a crucial component of the innate immune system, NK cells might have an important role in host defence against HIV infection, as well as in the control of HIV replication in vivo.
-
NK cells mediate suppression of viral replication in both a cytolytic manner and a non-cytolytic manner. The balance between the activation of inhibitory NK-cell receptors (iNKRs) and activating NK-cell receptors is fundamental to the regulation of NK-cell cytotoxic activity.
-
HIV is known to selectively downregulate the expression of MHC class I molecules at the surface of infected cells in vitro, thereby escaping recognition and lysis by CD8+ T cells and lysis by NK cells.
-
NK cells produce abundant amounts of CC-chemokines and have been shown to suppress HIV replication in vitro by inhibiting HIV entry to target cells. However, NK cells from individuals with HIV viraemia secrete reduced amounts of CC-chemokines and cannot adequately suppress HIV replication.
-
NK cells can eliminate infected cells during the effector phase of immune responses by mediating antibody-dependent cell-mediated cytotoxicity (ADCC). However, NK-cell-mediated ADCC is defective in HIV-infected individuals, which further compromises host immune defences against HIV.
-
The presence of the activating killer-cell immunoglobulin-like receptor (KIR) allele KIR3DS1 and the HLA-B allele HLA-Bw4 Ile80 is associated with delayed progression of HIV infection to AIDS, indicating that NK cells have a protective role against HIV-disease progression.
-
HIV viraemia induces several phenotypic and functional abnormalities in NK cells, including downregulation of expression of CD56 and activating NK-cell receptors, and upregulation of expression of iNKRs. Together, these changes contribute to the defective NK-cell function that has been described for HIV-infected individuals.
-
Expansion of a highly dysfunctional CD16hiCD56− NK-cell population in HIV-viraemic individuals is at least partially responsible for the observed defects in NK-cell function.
-
NK cells interact with dendritic cells (DCs) and regulate the maturation and function of DCs, thereby linking the innate and the adaptive immune responses. NK cells from HIV-infected individuals have decreased expression of NKG2A (NK group 2, member A) and NKp30 (NK-cell protein 30), as well as decreased secretion of interferon-γ, tumour-necrosis factor and granulocyte/macrophage colony-stimulating factor associated with the HIV-viraemic state, indicating that abnormal NK-cell–DC interactions occur in HIV-infected individuals.
-
Improvements in NK-cell function are observed with the initiation of antiretroviral therapy and the subsequent control of HIV viraemia.
Abstract
Natural killer cells are a crucial component of the innate immune response to certain tumours and to various viruses, fungi, parasites and bacteria. HIV has infected more than 60 million people worldwide and has led to more than 23 million deaths. At present, there are ∼40 million people who are living with HIV infection, and there were 5 million new infections in 2004. As part of the innate immune system, natural killer cells might have an important role in host defence against HIV infection, as well as in the control of HIV replication in vivo. In this regard, it is important to understand how natural killer cells and HIV interact. This Review focuses on the role of natural killer cells in controlling HIV infection and on the impact of HIV and HIV-viraemia-induced immune activation on natural-killer-cell function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
23 October 2005
When originally published, the thirteenth row in Table 1 contained incorrect information.
References
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Smyth, M. J., Godfrey, D. I. & Trapani, J. A. A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunol. 2, 293–299 (2001).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Moretta, A. et al. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 14, 619–648 (1996).
Long, E. O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17, 875–904 (1999).
Moretta, A. et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 (2001).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Moretta, A., Bottino, C., Mingari, M. C., Biassoni, R. & Moretta, L. What is a natural killer cell? Nature Immunol. 3, 6–8 (2002).
Cerwenka, A. & Lanier, L. L. Natural killer cells, viruses and cancer. Nature Rev. Immunol. 1, 41–49 (2001). This review describes the roles of signalling through activating and inhibitory receptors expressed by NK cells in the activity of NK cells against tumour cells and virus-infected cells.
Cooper, M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97, 3146–3151 (2001).
Cooper, M. A., Fehniger, T. A., Fuchs, A., Colonna, M. & Caligiuri, M. A. NK cell and DC interactions. Trends Immunol. 25, 47–52 (2004).
Nagler, A., Lanier, L. L., Cwirla, S. & Phillips, J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol. 143, 3183–3191 (1989).
Caligiuri, M. A. et al. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J. Exp. Med. 171, 1509–1526 (1990).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Scharton, T. M. & Scott, P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178, 567–577 (1993).
Orange, J. S., Wang, B., Terhorst, C. & Biron, C. A. Requirement for natural killer cell-produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182, 1045–1056 (1995).
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Degli-Esposti, M. A. & Smyth, M. J. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nature Rev. Immunol. 5, 112–124 (2005).
Moretta, A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nature Rev. Immunol. 2, 957–964 (2002).
Zitvogel, L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 195, F9–F14 (2002).
Brenner, B. G., Dascal, A., Margolese, R. G. & Wainberg, M. A. Natural killer cell function in patients with acquired immunodeficiency syndrome and related diseases. J. Leukoc. Biol. 46, 75–83 (1989).
Bonaparte, M. I. & Barker, E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS 17, 487–494 (2003).
Bonaparte, M. I. & Barker, E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104, 2087–2094 (2004). This study shows that the capacity of NK cells to lyse HIV-infected target cells in vitro depends on the ability of the virus to modulate expression of MHC class I molecules.
Tasca, S. et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS 17, 2291–2298 (2003). This study describes impaired NK-cell-mediated killing of immature DCs in the presence of HIV viraemia.
Rook, A. H. et al. Interleukin-2 enhances the depressed natural killer and cytomegalovirus-specific cytotoxic activities of lymphocytes from patients with the acquired immune deficiency syndrome. J. Clin. Invest. 72, 398–403 (1983).
Rook, A. H. et al. Interleukin 2 enhances the natural killer cell activity of acquired immunodeficiency syndrome patients through a γ-interferon-independent mechanism. J. Immunol. 134, 1503–1507 (1985).
Ahmad, A. & Menezes, J. Defective killing activity against gp120/41-expressing human erythroleukaemic K562 cell line by monocytes and natural killer cells from HIV-infected individuals. AIDS 10, 143–149 (1996).
Ahmad, A. & Menezes, J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10, 258–266 (1996).
Brenner, B. G., Gryllis, C. & Wainberg, M. A. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. J. Leukoc. Biol. 50, 628–640 (1991).
Masur, H., Kaplan, J. E. & Holmes, K. K. Guidelines for preventing opportunistic infections among HIV-infected persons — 2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann. Intern. Med. 137, 435–478 (2002).
Bandyopadhyay, S. et al. Natural killer cell-mediated lysis of T cell lines chronically infected with HIV-1. Clin. Exp. Immunol. 79, 430–435 (1990).
Sirianni, M. C. et al. Natural killer activity from normal peripheral blood lymphocytes against a human T lymphotropic retrovirus type III (HTLV-III)-infected cell line. Diagn. Clin. Immunol. 5, 297–303 (1988).
Fortis, C. et al. Dual role of TNF-α in NK/LAK cell-mediated lysis of chronically HIV-infected U1 cells. Concomitant enhancement of HIV expression and sensitization of cell-mediated lysis. Eur. J. Immunol. 29, 3654–3662 (1999).
Ward, J. P., Bonaparte, M. I. & Barker, E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 18, 1769–1779 (2004).
Cohen, G. B. et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10, 661–671 (1999). This study shows that selective downregulation of expression of MHC class I molecules by HIV-infected cells enables these cells to escape killing by cytotoxic T lymphocytes while maintaining protection from NK-cell killing.
Mavilio, D. et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl Acad. Sci. USA 100, 15011–15016 (2003). This is a comprehensive study analysing the effect of HIV viraemia on the cell-surface expression and function of activating and inhibitory receptors at the surface of NK cells.
Kottilil, S. Natural killer cells in HIV-1 infection: role of NK cell-mediated non-cytolytic mechanisms in pathogenesis of HIV-1 infection. Indian J. Exp. Biol. 41, 1219–1225 (2003).
Choe, H. et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 (1996).
Dragic, T. et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 (1996).
Alkhatib, G. et al. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 (1996).
Bluman, E. M., Bartynski, K. J., Avalos, B. R. & Caligiuri, M. A. Human natural killer cells produce abundant macrophage inflammatory protein-1α in response to monocyte-derived cytokines. J. Clin. Invest. 97, 2722–2727 (1996).
Fehniger, T. A. et al. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J. Immunol. 161, 6433–6438 (1998).
Kottilil, S. et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187, 1038–1045 (2003). This study shows the effect of HIV viraemia on the ability of NK cells to secrete CC-chemokines and suppress HIV replication in vitro.
Oliva, A. et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Invest. 102, 223–231 (1998).
Scott-Algara, D. et al. Increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 171, 5663–5667 (2003). This study describes a potential role for NK cells in protecting individuals who are at high risk of HIV infection from acquiring HIV infection.
Follezou, J. Y. et al. Clinical and biological characteristics of human immunodeficiency virus-infected and uninfected intravascular drug users in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 61, 420–424 (1999).
Dokun, A. O. et al. Specific and nonspecific NK cell activation during virus infection. Nature Immunol. 2, 951–956 (2001).
Martin, M. P. et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature Genet. 31, 429–434 (2002). This study shows that the presence of the activating allele KIR3DS1 and of HLA-Bw4 Ile80 is associated with delayed progression to AIDS in individuals who are infected with HIV.
Flores-Villanueva, P. O. et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl Acad. Sci. USA 98, 5140–5145 (2001).
Kottilil, S. et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J. Infect. Dis. 189, 1193–1198 (2004).
Hu, P. F. et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10, 331–340 (1995).
Lucia, B., Jennings, C., Cauda, R., Ortona, L. & Landay, A. L. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry 22, 10–15 (1995).
Tarazona, R. et al. Selective depletion of CD56dim NK cell subsets and maintenance of CD56bright NK cells in treatment-naive HIV-1-seropositive individuals. J. Clin. Immunol. 22, 176–183 (2002).
Valentin, A. et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 99, 7015–7020 (2002). This is the first study to show that a subset of NK cells can be productively infected with HIV and that these cells might be a latent reservoir of HIV in vivo.
Arthos, J. et al. The role of the CD4 receptor versus HIV coreceptors in envelope-mediated apoptosis in peripheral blood mononuclear cells. Virology 292, 98–106 (2002).
Cicala, C. et al. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J. Immunol. 163, 420–426 (1999).
Ahmad, R. et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21, 227–233 (2001).
Parato, K. G. et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS 16, 1251–1256 (2002).
Sirianni, M. C. et al. Distribution of the natural killer-related receptor for HLA-C during highly active antiretroviral therapy for human immunodeficiency virus infection. Hum. Immunol. 62, 1328–1334 (2001).
Ahmad, R. et al. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J. Med. Virol. 65, 431–440 (2001).
Scott-Algara, D., Vuillier, F., Cayota, A. & Dighiero, G. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin. Exp. Immunol. 90, 181–187 (1992).
De Maria, A. et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol. 33, 2410–2418 (2003).
Fogli, M. et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur. J. Immunol. 34, 2313–2321 (2004).
Mavilio, D. et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl Acad. Sci. USA 102, 2886–2891 (2005).
Scott-Algara, D. & Paul, P. NK cells and HIV infection: lessons from other viruses. Curr. Mol. Med. 2, 757–768 (2002).
Sondergaard, S. R. et al. Immune function and phenotype before and after highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 21, 376–383 (1999).
Sondergaard, S. R., Ullum, H. & Pedersen, B. K. Proliferative and cytotoxic capabilities of CD16+CD56− and CD16+/−CD56+ natural killer cells. APMIS 108, 831–837 (2000).
Sirianni, M. C., Mezzaroma, I., Aiuti, F. & Moretta, A. Analysis of the cytolytic activity mediated by natural killer cells from acquired immunodeficiency syndrome patients in response to phytohemagglutinin or anti-CD16 monoclonal antibody. Eur. J. Immunol. 24, 1874–1878 (1994).
Ullum, H. et al. Defective natural immunity: an early manifestation of human immunodeficiency virus infection. J. Exp. Med. 182, 789–799 (1995). In this study, the authors observed that defects in cytokine responsiveness of NK cells, particularly to IFN-γ, occurred during the early stages of HIV infection.
Ullum, H. et al. Natural immunity and HIV disease progression. AIDS 13, 557–563 (1999).
Ahmad, A., Morisset, R., Thomas, R. & Menezes, J. Evidence for a defect of antibody-dependent cellular cytotoxic (ADCC) effector function and anti-HIV gp120/41-specific ADCC-mediating antibody titres in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 7, 428–437 (1994).
Azzoni, L. et al. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 168, 5764–5770 (2002).
Lin, S. J. et al. Effect of interleukin (IL)-12 and IL-15 on activated natural killer (ANK) and antibody-dependent cellular cytotoxicity (ADCC) in HIV infection. J. Clin. Immunol. 18, 335–345 (1998).
Lum, J. J. et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J. Virol. 78, 6033–6042 (2004).
Loubeau, M., Ahmad, A., Toma, E. & Menezes, J. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16, 137–145 (1997).
Alter, G., Malenfant, J. M. & Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294, 15–22 (2004).
Azzoni, L. et al. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1-infected children. J. Infect. Dis. 191, 1451–1459 (2005).
Raulet, D. H. Interplay of natural killer cells and their receptors with the adaptive immune response. Nature Immunol. 5, 996–1002 (2004).
Della Chiesa, M., Sivori, S., Castriconi, R., Marcenaro, E. & Moretta, A. Pathogen-induced private conversations between natural killer and dendritic cells. Trends Microbiol. 13, 128–136 (2005).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nature Immunol. 5, 1260–1265 (2004).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Della Chiesa, M. et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur. J. Immunol. 33, 1657–1666 (2003).
Vitale, M. et al. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood 106, 566–571 (2005).
Biassoni, R. et al. Molecular and functional characterization of NKG2D, NKp80, and NKG2C triggering NK cell receptors in rhesus and cynomolgus macaques: monitoring of NK cell function during simian HIV infection. J. Immunol. 174, 5695–5705 (2005).
Carter, D. L. et al. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry 37, 41–50 (1999).
Mavilio, D. et al. Identification of NKG2A and NKp80 as specific natural killer cell markers in rhesus and pig-tailed monkeys. Blood 106, 1718–1725 (2005).
Gupta, N. et al. Targeted lysis of HIV-infected cells by natural killer cells armed and triggered by a recombinant immunoglobulin fusion protein: implications for immunotherapy. Virology 332, 491–497 (2005).
Sivori, S. et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl Acad. Sci. USA 101, 10116–10121 (2004).
Ashkar, A. A., Bauer, S., Mitchell, W. J., Vieira, J. & Rosenthal, K. L. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J. Virol. 77, 8948–8956 (2003). This study shows that local induction of mucosal innate immunity can provide protection against sexually transmitted infections, such as that caused by herpes simplex virus 2, at mucosal surfaces.
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Colonna, M. et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818 (1997).
Moretta, A., Biassoni, R., Bottino, C., Mingari, M. C. & Moretta, L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol. Today 21, 228–234 (2000).
Bottino, C., Castriconi, R., Moretta, L. & Moretta, A. Cellular ligands of activating NK receptors. Trends Immunol. 26, 221–226 (2005).
Daniels, K. A. et al. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194, 29–44 (2001).
Smith, H. R. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl Acad. Sci. USA 99, 8826–8831 (2002).
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002).
Mandelboim, O. et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409, 1055–1060 (2001).
Barclay, A. et al. (eds) The Leucocyte Antigen Facts Book (Academic, San Diego, 1997).
Falco, M. et al. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 190, 793–802 (1999).
Acknowledgements
The authors acknowledge A. Moretta for critical comment on the manuscript, J. Weddle for assistance with figure designs, and J. Jackson and D. Kim for appraising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ANTIBODY-DEPENDENT CELL-MEDIATED CYTOTOXICITY
-
(ADCC). A cytotoxic mechanism by which an antibody-coated target cell is directly killed by a leukocyte that expresses Fc receptors, such as a natural killer (NK) cell, macrophage or neutrophil. A specific receptor for the constant region of IgG, FcγRIII (also known as CD16), is expressed at the surface of most NK cells and mediates ADCC.
- ANTIRETROVIRAL THERAPY
-
(ART). Combination treatment regimens of antiretroviral drugs that effectively suppress HIV replication and delay progression to AIDS. In general, ART includes three or more drugs, such as two nucleoside reverse-transcriptase inhibitors (NRTIs), one protease inhibitor and/or one non-NRTI. One new class of antiretroviral agent is a fusion inhibitor (enfuvirtide), which blocks the entry of HIV to cells, and it is generally used to treat individuals who are infected with multidrug-resistant HIV.
- R5 VIRUS
-
An HIV strain that uses CC-chemokine receptor 5 (CCR5) as the co-receptor to gain entry to target cells.
- X4 VIRUS
-
An HIV strain that uses CXC-receptor 4 (CXCR4) as the co-receptor to gain entry to target cells.
- MOUSE CYTOMEGALOVIRUS
-
(MCMV). Immune responses to this herpesvirus-family member can suppress viral replication but do not completely eliminate the virus, leading to persistent infection. MCMV is highly homologous to human cytomegalovirus and is therefore often used as an in vivo model of chronic viral infection in a natural host.
- SEROCONVERSION
-
The development of antibodies specific for HIV antigens. When individuals develop antibodies specific for HIV, they seroconvert from an antibody-negative state to an antibody-positive state. After infection with HIV, it might take from as little as 1 week to several months (or more) for antibodies to the virus to develop.
- EPISTATIC ASSOCIATION
-
In genetic epidemiology, an epistatic effect is the modification of the risk that is conferred by one marker by the presence of a marker from an unrelated gene (that is, an unlinked gene–gene interaction).
- TOLL-LIKE RECEPTORS
-
(TLRs). A family of receptors that are homologous to Drosophila melanogaster Toll. TLRs recognize conserved molecular patterns that are present in pathogens, such as lipopolysaccharide in the bacterial cell wall.
- CPG-CONTAINING OLIGODEOXYNUCLEOTIDE
-
(CpG ODN). An ODN that includes a cytosine residue joined by a 5′-to-3′ phosphodiester linkage to a guanine residue as part of a normal DNA strand. This sequence is highly immunogenic and induces an innate immune response through interaction with Toll-like receptor 9.
Rights and permissions
About this article
Cite this article
Fauci, A., Mavilio, D. & Kottilil, S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat Rev Immunol 5, 835–843 (2005). https://doi.org/10.1038/nri1711
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1711
This article is cited by
-
NK cells from Men Who Have Sex with Men at high risk for HIV-1 infection exhibit higher effector capacity
Scientific Reports (2023)
-
IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours
Nature Communications (2017)
-
Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV
Protein & Cell (2017)
-
Increased CD56bright NK cells in HIV-HCV co-infection and HCV mono-infection are associated with distinctive alterations of their phenotype
Virology Journal (2016)
-
Natural killer cell heterogeneity: cellular dysfunction and significance in HIV-1 immuno-pathogenesis
Cellular and Molecular Life Sciences (2015)