Key Points
-
Animal studies have demonstrated that changes in the gut microbiota result in altered host function, in domains relevant to IBS (gut motility, visceral pain responses, intestinal permeability, and brain function and behaviour)
-
Gut microbiota composition is altered in at least a subset of patients with IBS (most commonly diarrhoea-predominant IBS), but no microbial 'signature' that could act as an IBS biomarker has been identified
-
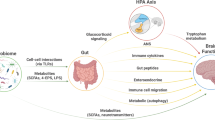
Considerable interest exists in the ability of bacteria to produce substances that interact with the host to influence gut and brain function, which include fatty acids, tryptophan and neurotransmitters
-
Dysbiosis in IBS is characterized by a loss of microbial diversity and temporal instability; contributing factors include diet, stress, infection, antibiotic usage, immune activation and low-grade inflammation
-
The gut microbiota from patients with IBS, but not healthy individuals, can induce gut dysfunction in mice reminiscent of that seen in IBS, strongly suggesting that the microbiota contributes to the expression of IBS
-
Emerging evidence supports the efficacy of select and limited microbiota-directed therapies in treating IBS, and to date these include prebiotics, probiotics and selected antibiotics
Abstract
The past decade has witnessed an explosion of knowledge regarding the vast microbial community that resides within our intestine—the gut microbiota. The topic has generated great expectations in terms of gaining a better understanding of disorders ranging from IBD to metabolic disorders and obesity. IBS is a condition for which investigators have long been in search of plausible underlying pathogeneses and it is inevitable that altered composition or function of the gut microbiota will be considered as a potential aetiological factor in at least a subset of patients with IBS. This Review describes the evidence implicating the gut microbiota in not only the expression of the intestinal manifestations of IBS, but also the psychiatric morbidity that coexists in up to 80% of patients with IBS. The evidence described herein ranges from proof-of-concept studies in animals to observational studies and clinical trials in humans. The gut microbiota is subject to influences from a diverse range of factors including diet, antibiotic usage, infection and stress. These factors have previously been implicated in the pathophysiology of IBS and further prompt consideration of a role for the gut microbiota in IBS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Sekirov, I., Russell, S. L., Antunes, L. C. & Finlay, B. B. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Shanahan, F. The colonic microbiota in health and disease. Curr. Opin. Gastroenterol. 29, 49–54 (2013).
Rajilic-Stojanovic, M. Function of the microbiota. Best Pract Res. Clin. Gastroenterol. 27, 5–16 (2013).
Jorgensen, J. & Mortensen, P. B. Utilization of short-chain fatty acids by colonic mucosal tissue strips. A new method of assessing colonic mucosal metabolism. Scand. J. Gastroenterol. 35, 659–666 (2000).
Barrett, E., Ross, R. P., O'Toole, P. W., Fitzgerald, G. F. & Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417 (2012).
McDermott, A. J. & Huffnagle, G. B. The microbiome and regulation of mucosal immunity. Immunology. http://dx.doi.org/10.1111/imm.12231.
Chu, H. & Mazmanian, S. K. Innate immune recognition of the microbiota promotes host–microbial symbiosis. Nat. Immunol. 14, 668–675 (2013).
Gwee, K. A. et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 347, 150–153 (1996).
Marshall, J. K. et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 131, 445–450 (2006).
Neal, K. R., Barker, L. & Spiller, R. C. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut 51, 410–413 (2002).
Spiller, R. C. Inflammation as a basis for functional GI disorders. Best Pract. Res. Clin. Gastroenterol. 18, 641–661 (2004).
Cremonini, F. & Talley, N. J. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol. Clin. North Am. 34, 189–204 (2005).
Salonen, A., de Vos, W. M. & Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology 156, 3205–3215 (2010).
Jeffery, I. B., Quigley, E. M., Ohman, L., Simren, M. & O'Toole, P. W. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes 3, 572–576 (2012).
Durban, A. et al. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol. Ecol. 86, 581–589 (2013).
Matto, J. et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome—a longitudinal study in IBS and control subjects. FEMS Immunol. Med. Microbiol. 43, 213–222 (2005).
Maukonen, J. et al. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J. Med. Microbiol. 55, 625–633 (2006).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Rajilic-Stojanovic, M. et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141, 1792–1801 (2011).
Bourdu, S. et al. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 128, 1996–2008 (2005).
Kamath, P. S., Phillips, S. F. & Zinsmeister, A. R. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology 95, 1496–1502 (1988).
Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 22, 512–519 (2010).
Christl, S. U., Murgatroyd, P. R., Gibson, G. R. & Cummings, J. H. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology 102, 1269–1277 (1992).
Dridi, B., Raoult, D. & Drancourt, M. Archaea as emerging organisms in complex human microbiomes. Anaerobe 17, 56–63 (2011).
Kim, G. et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig. Dis. Sci. 57, 3213–3218 (2012).
Pimentel, M. et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1089–G1095 (2006).
Furnari, M. et al. Reassessment of the role of methane production between irritable bowel syndrome and functional constipation. J. Gastrointestin. Liver Dis. 21, 157–163 (2012).
Pimentel, M. et al. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig. Dis. Sci. 48, 86–92 (2003).
Abrahamsson, H. Gastrointestinal motility in patients with the irritable bowel syndrome. Scand. J. Gastroenterol. Suppl 130, 21–26 (1987).
Pimentel, M., Chow, E. J. & Lin, H. C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 95, 3503–3506 (2000).
Pimentel, M., Soffer, E. E., Chow, E. J., Kong, Y. & Lin, H. C. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig. Dis. Sci. 47, 2639–2643 (2002).
Pyleris, E. et al. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Dig. Dis. Sci. 57, 1321–1329 (2012).
Posserud, I., Stotzer, P. O., Bjornsson, E. S., Abrahamsson, H. & Simren, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56, 802–808 (2007).
Spiegel, B. M. Questioning the bacterial overgrowth hypothesis of irritable bowel syndrome: an epidemiologic and evolutionary perspective. Clin. Gastroenterol. Hepatol. 9, 461–469 (2011).
Husebye, E., Hellstrom, P. M., Sundler, F., Chen, J. & Midtvedt, T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G368–G380 (2001).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Anitha, M., Vijay-Kumar, M., Sitaraman, S. V., Gewirtz, A. T. & Srinivasan, S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143, 1006–1016 (2012).
Verdu, E. F. et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55, 182–190 (2006).
Ohman, L. & Simren, M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 7, 163–173 (2010).
Collins, S. M. Is the irritable gut an inflamed gut? Scand. J. Gastroenterol. Suppl. 192, 102–105 (1992).
Ford, A. C. & Talley, N. J. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J. Gastroenterol. 46, 421–431 (2011).
Lyra, A. et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 15, 5936–5945 (2009).
Neufeld, K. M., Kang, N., Bienenstock, J. & Foster, J. A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264 (2011).
Diaz Heijtz, R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).
Li, W., Dowd, S. E., Scurlock, B., Acosta-Martinez, V. & Lyte, M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 96, 557–567 (2009).
Bercik, P. et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609 (2011).
Collins, S. M., Kassam, Z. & Bercik, P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr. Opin. Microbiol. 16, 240–245 (2013).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 204 (2007).
Hoffmann, C. et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 77, 4668–4678 (2009).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009).
Villani, A. C. et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology 138, 1502–1513 (2010).
Marshall, J. K. et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 20, 1317–1322 (2004).
Spiller, R. C. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811 (2000).
Gwee, K. A. et al. Increased rectal mucosal expression of interleukin 1β in recently acquired post-infectious irritable bowel syndrome. Gut 52, 523–526 (2003).
Berman, S. et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage 63, 1854–1863 (2012).
Bailey, M. T. & Coe, C. L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35, 146–155 (1999).
Bailey, M. T. et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407 (2011).
Knowles, S. R., Nelson, E. A. & Palombo, E. A. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol. Psychol. 77, 132–137 (2008).
Park, A. J. et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 25, 733–e575 (2013).
Heitkemper, M. et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am. J. Gastroenterol. 91, 906–913 (1996).
Lyte, M., Vulchanova, L. & Brown, D. R. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 343, 23–32 (2011).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4, 158–164 (2013).
Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Dethlefsen, L. & Relman, D. A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4554–4561 (2011).
Jernberg, C., Lofmark, S., Edlund, C. & Jansson, J. K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156, 3216–3223 (2010).
Mendall, M. A. & Kumar, D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur. J. Gastroenterol. Hepatol. 10, 59–62 (1998).
Villarreal, A. A., Aberger, F. J., Benrud, R. & Gundrum, J. D. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ 111, 17–20 (2012).
Bohn, L., Storsrud, S., Tornblom, H., Bengtsson, U. & Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 108, 634–641 (2013).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Simren, M., Abrahamsson, H. & Bjornsson, E. S. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut 48, 20–27 (2001).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487, 104–108 (2012).
Barkhordari, E. et al. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J. Clin. Immunol. 30, 74–79 (2010).
Gonsalkorale, W. M., Perrey, C., Pravica, V., Whorwell, P. J. & Hutchinson, I. V. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut 52, 91–93 (2003).
van der Veek, P. P., van den Berg, M., de Kroon, Y. E., Verspaget, H. W. & Masclee, A. A. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am. J. Gastroenterol. 100, 2510–2516 (2005).
Ong, D. K. et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 25, 1366–1373 (2010).
Hoveyda, N. et al. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 9, 15 (2009).
McFarland, L. V. & Dublin, S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J. Gastroenterol. 14, 2650–2661 (2008).
Moayyedi, P. et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59, 325–332 (2010).
Ortiz-Lucas, M., Tobias, A., Saz, P. & Sebastian, J. J. Effect of probiotic species on irritable bowel syndrome symptoms: a bring up to date meta-analysis. Rev. Esp. Enferm. Dig. 105, 19–36 (2013).
Whelan, K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care 14, 581–587 (2011).
Aureli, P. et al. Probiotics and health: an evidence-based review. Pharmacol. Res. 63, 366–376 (2011).
Corridoni, D. et al. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS ONE 7, e42067 (2012).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Vitali, B. et al. An in vitro evaluation of the effect of probiotics and prebiotics on the metabolic profile of human microbiota. Anaerobe 18, 386–391 (2012).
Brasili, E. et al. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 induce different age-related metabolic profiles revealed by 1H-NMR spectroscopy in urine and feces of mice. J. Nutr. 143, 1549–57 (2013).
Cox, M. J. et al. Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PLoS ONE 5, e8745 (2010).
Ki Cha, B. et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 46, 220–227 (2012).
Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104 (Suppl. 2), S1–S63 (2010).
Dewulf, E. M. et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 (2013).
Silk, D. B., Davis, A., Vulevic, J., Tzortzis, G. & Gibson, G. R. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 29, 508–518 (2009).
Halmos, E. P., Power, V. A., Shepherd, S. J., Gibson, P. R. & Muir, J. G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75 (2014).
Darkoh, C. et al. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob. Agents Chemother. 54, 3618–3624 (2010).
Menees, S. B., Maneerattannaporn, M., Kim, H. M. & Chey, W. D. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am. J. Gastroenterol. 107, 28–35 (2012).
Pimentel, M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin. Investig. Drugs 18, 349–358 (2009).
Collins, S. M. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 111, 1683–1699 (1996).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Nutten, S. et al. Antibiotic administration early in life impairs specific humoral responses to an oral antigen and increases intestinal mast cell numbers and mediator concentrations. Clin. Vaccine Immunol. 14, 190–197 (2007).
Crouzet, L. et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 25, e272–e282 (2013).
Ohman, L., Isaksson, S., Lundgren, A., Simren, M. & Sjovall, H. A controlled study of colonic immune activity and β7+ blood T lymphocytes in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 3, 980–986 (2005).
Rodriguez-Fandino, O. et al. Intestinal recruiting and activation profiles in peripheral blood mononuclear cells in response to pathogen-associated molecular patterns stimulation in patients with IBS. Neurogastroenterol. Motil. 25, 872–e699 (2013).
Brint, E. K., MacSharry, J., Fanning, A., Shanahan, F. & Quigley, E. M. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 106, 329–336 (2011).
Belmonte, L. et al. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS ONE 7, e42777 (2012).
McKernan, D. P., Gaszner, G., Quigley, E. M., Cryan, J. F. & Dinan, T. G. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 33, 1045–1052 (2011).
Langhorst, J. et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am. J. Gastroenterol. 104, 404–410 (2009).
Macsharry, J. et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand. J. Gastroenterol. 43, 1467–1476 (2008).
Bashashati, M. et al. Cytokine gene polymorphisms are associated with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 24, 1102–e566 (2012).
O'Mahony, L. et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128, 541–551 (2005).
Ness, T. J. & Gebhart, G. F. Visceral pain: a review of experimental studies. Pain 41, 167–234 (1990).
Chassard, C. et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 35, 828–838 (2012).
Bercik, P. et al. Transfer of IBS phenotype to GERM-free mice through microbiota transplantation. Neurogastroenterol. Motility 24, 33–33 (2012).
Kashyap, P. C. et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144, 967–977 (2013).
Freestone, P. P., Sandrini, S. M., Haigh, R. D. & Lyte, M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 16, 55–64 (2008).
Ouwerkerk, J. P., de Vos, W. M. & Belzer, C. Glycobiome: bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 27, 25–38 (2013).
Collins, S. M., Surette, M. & Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735–742 (2012).
Acknowledgements
S.M.C. acknowledges the Canadian Institutes for Health Research for support of his research cited in this Review. S.M.C. is the recipient of the GlaxoSmithKline Chair in Gastroenterology Research and a founding member of the Farncombe Family Digestive Health Research Institute at McMaster University, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.M.C. receives a grant in aid from the Nestle Research Centre, Switzerland, and consults for Salix Inc.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Collins, S. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol 11, 497–505 (2014). https://doi.org/10.1038/nrgastro.2014.40
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.40
This article is cited by
-
Alterations of the gut microbiome and metabolic profile in CVB3-induced mice acute viral myocarditis
BMC Microbiology (2023)
-
Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome and irritable bowel syndrome
Nature Communications (2023)
-
The critical role of gut-brain axis microbiome in mental disorders
Metabolic Brain Disease (2023)
-
Fecal Microbiota Signatures Are Not Consistently Related to Symptom Severity in Irritable Bowel Syndrome
Digestive Diseases and Sciences (2022)
-
Distinctions Between Fecal and Intestinal Mucosal Microbiota in Subgroups of Irritable Bowel Syndrome
Digestive Diseases and Sciences (2022)