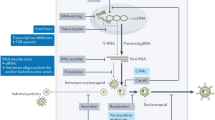
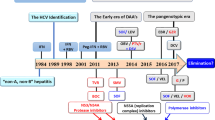
Abstract
The goal of hepatitis B treatment is to prevent cirrhosis, liver decompensation and hepatocellular carcinoma. In clinical practice, treatment response is determined by suppression of serum HBV DNA levels, hepatitis B e antigen seroconversion to hepatitis B e antibody, hepatitis B surface antigen loss, normalization of alanine aminotransferase levels and improvement in liver histology. Patients with life-threatening liver disease, and those with high levels of HBV replication and active or advanced liver disease, should be treated. Other patients should be monitored so that treatment can be initiated when indicated. Currently, seven medications are approved for the treatment of hepatitis B: two formulations of interferon and five nucleos(t)ide analogues. Interferon is administered for a finite duration while nucleos(t)ide analogues are usually administered for many years. Antiviral drug resistance is a major limiting factor to the success of nucleos(t)ide analogue treatment; therefore, treatment should be initiated with drugs that have a high genetic barrier to resistance (that is, a low potential for drug resistance). In addition, treatment response should be closely monitored to detect virologic breakthroughs, and the importance of medication adherence should be emphasized. Management of patients with treatment failure should be tailored according to the type of treatment failure (lack of initial response versus virologic breakthrough), the treatment that the patient is receiving, history of prior treatment, and the pretreatment characteristics of both the patient and the disease.
Key Points
-
Indication for hepatitis B treatment is determined by HBV replication status and the activity and stage of liver disease
-
Patient age, hepatitis B e antigen status, and other circumstances—such as family history of HCC, occupational requirement, need for immunosuppressive or cancer chemotherapy, and pregnancy plans—should also be considered when deciding on treatment
-
Patients with chronic HBV infection need life-long monitoring because the status of the disease can change with time
-
There are seven approved treatments for hepatitis B: two formulations of interferon (conventional and pegylated), and five nucleos(t)ide analogues (lamivudine, entecavir, tenofovir disoproxil, adefovir dipivoxil and telbivudine)
-
PEG-IFN, entecavir and tenofovir disoproxil are the preferred first-line treatments for hepatitis B
-
Antiviral drug resistance is a major limiting factor to the success of nucleos(t)ide analogue therapy; treatment should be initiated with drugs that have a high genetic barrier to resistance and virologic response should be closely monitored
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yim, H. J. & Lok, A. S. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 43, S173–S181 (2006).
Fattovich, G., Bortolotti, F. & Donato, F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J. Hepatol. 48, 335–352 (2008).
Chu, C. M., Hung., S. J., Lin, J., Tai, D. I. & Liaw, Y. F. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am. J. Med. 116, 829–834 (2004).
Liaw, Y. F. et al. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology 84, 216–219 (1983).
Lok, A. S., Lai, C. L., Wu, P. C., Leung, E. K. & Lam, T. S. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology 92, 1839–1843 (1987).
Chen, Y. C., Chu, C. M. & Liaw, Y. F. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 51, 435–444 (2010).
Manno, M. et al. Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology 127, 756–763 (2004).
Chotiyaputta, W. & Lok, A. S. Hepatitis B virus variants. Nat. Rev. Gastroenterol. Hepatol. 6, 453–462 (2009).
Iloeje, U. et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130, 678–686 (2006).
Chen, C. J. et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295, 65–73 (2006).
van Zonneveld, M. et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology 39, 804–810 (2004).
Niederau, C. et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N. Engl. J. Med. 334, 1422–1427 (1996).
Brunetto, M. R. et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J. Hepatol. 36, 263–270 (2002).
Papatheodoridis, G. V., Manesis, E. & Hadziyannis, S. J. The long-term outcome of interferon-alpha treated and untreated patients with HBeAg-negative chronic hepatitis B. J. Hepatol. 34, 306–313 (2001).
Lampertico, P. et al. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology 37, 756–763 (2003).
Lau, G. K. et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 352, 2682–2695 (2005).
Janssen, H. L. et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365, 123–129 (2005).
Chan, H. L. et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann. Intern. Med. 142, 240–250 (2005).
Buster, E. H. et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon a-2b. Gastroenterology 135, 459–467 (2008).
Marcellin, P. et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 351, 1206–1217 (2004).
Marcellin, P. et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 136, 2169–2179.e1–4 (2009).
Perrillo, R. P. et al. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology 36, 186–194 (2002).
Wai, C. T., Chu, C. J., Hussain, M. & Lok, A. S. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology 36, 1425–1430 (2002).
Buster, E. H. et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 137, 2002–2009 (2009).
Rijckborst, V. et al. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology 52, 454–461 (2010).
Moucari, R. et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 49, 1151–1157 (2009).
Hoofnagle, J. H., Di Bisceglie, A. M., Waggoner, J. G. & Park, Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology 104, 1116–1121 (1993).
Buster, E. H. et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology 46, 388–394 (2007).
Dienstag, J. L. et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 341, 1256–1263 (1999).
Lai, C. et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N. Engl. J. Med. 339, 61–68 (1998).
Marcellin, P. et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348, 808–816 (2003).
Chang, T. T. et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 354, 1001–1010 (2006).
Marcellin, P. et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 359, 2442–2455 (2008).
Lai, C. L. et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 357, 2576–2588 (2007).
Lee, H. W. et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology 51, 415–421 (2010).
Lok, A. S. et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 125, 1714–1722 (2003).
Marcellin, P. et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology 48, 750–758 (2008).
Chang, T. T. et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 51, 422–430 (2010).
Liaw, Y. F. et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 136, 486–495 (2009).
Heathcote, E. J. et al. Long term (4 year) efficacy and safety of tenofovir disoproxil fumarate (TDF) treatment in HBeAg-positive patients (HBEAG+) with chronic hepatitis B (Study 103): preliminary analysis (abstract 477). Hepatology 52, 556A–557A (2010).
Dienstag, J. L. et al. Histological outcome during long-term lamivudine therapy. Gastroenterology 124, 105–117 (2003).
Hadziyannis, S. et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 131, 1743–1751 (2006).
Chang, T. T. et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52, 886–893 (2010).
Tassopoulos, N. C. et al. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology 29, 889–896 (1999).
Hadziyannis, S. J. et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348, 800–807 (2003).
Lai, C. L. et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 354, 1011–1020 (2006).
Marcellin, P. et al. Continued efficacy and safety through 4 years of tenofovir disoproxil fumarate (TDF) treatment in HBeAg-negative patients with chronic hepatitis B (study 102): preliminary analysis (abstract 476). Hepatology 52, 555A–556A (2010).
Lok, A. S. et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology 46, 254–265 (2007).
Tenney, D. J. et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 49, 1503–1514 (2009).
Tenney, D. J. et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob. Agents Chemother. 48, 3498–3507 (2004).
Rapti, I., Dimou, E., Mitsoula, P. & Hadziyannis, S. J. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 45, 307–313 (2007).
Yim, H. J. et al. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 44, 703–712 (2006).
Wu, I. C. et al. Efficacy of entecavir in chronic hepatitis B patients with mildly elevated alanine aminotransferase and biopsy-proven histological damage. Hepatology 51, 1185–1189 (2010).
Westland, C. et al. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil1. Gastroenterology 125, 107–116 (2003).
Verhelst, D. et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am. J. Kidney Dis. 40, 1331–1333 (2002).
Purdy, J. B., Gafni, R. I., Reynolds, J. C., Zeichner, S. & Hazra, R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J. Pediatr. 152, 582–584 (2008).
Lange, C. M. et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 50, 2001–2006 (2009).
Lok, A. S. & McMahon, B. J. Practice guidelines: chronic hepatitis B. Hepatology 45, 507–539 (2007).
Lok, A. S. & McMahon, B. J. Chronic hepatitis B: update 2009. American Association for the Study of Liver Diseases [online], (2009).
Sorrell, M. F. et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann. Intern. Med. 150, 104–110 (2009).
EASL Clinical Practice Guidelines: management of chronic hepatitis B. J. Hepatol. 50, 227–242 (2009).
Liaw, Y. F. et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol. Int. 2, 263–283 (2008).
Fontana, R. J. et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 123, 719–727 (2002).
Yao, F. Y., Terrault, N. A., Freise, C., Maslow, L. & Bass, N. M. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology 34, 411–416 (2001).
Liaw, Y. F. et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 351, 1521–1531 (2004).
Hoofnagle, J. H. Reactivation of hepatitis B. Hepatology 49, S156–S165 (2009).
Loomba, R. et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann. Intern. Med. 148, 519–528 (2008).
Shi, Z., Yang, Y., Ma, L., Li, X. & Schreiber, A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet. Gynecol. 116, 147–159 (2010).
Han, G., Zhao, W., Cao, M., Jiang, H. & Pan, C. A prospective and open-label study for the efficacy and safety of telbivudine(LTD) in pregnancy for the prevention of perinatal transmission of hepatitis B virus (HBV) to the infants (abstract 212). Hepatology 52, 427A–428A (2010).
Reijnders, J. G., Perquin, M. J., Zhang, N., Hansen, B. E. & Janssen, H. L. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology 139, 491–498 (2010).
Sung, J. J. et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J. Hepatol. 48, 728–735 (2008).
Lai, C. L. et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 129, 528–536 (2005).
Berg, T. et al. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology 139, 1207–1217 (2010).
Liaw, Y. et al. Shorter duration and lower dose of Peginterferon Alfa-2A therapy results in inferior HBeAg seroconversion rates compared with the duration and dose of 48 weeks and 180 mG: neptune study (abstract 215). Hepatology 521, 429A–430A (2010).
Fan, X. et al. A head-to-head comparison of peginterferon α-2B treatment regimens in the treatment of Chinese and south-east Asian patients with HBeAg positive chronic hepatitis B. Hepatology 52, 386A (2010).
Lampertico, P. et al. Safety and tolerability of extended (96 weeks) treatment with peginterferon alfa-2A [40KD] in genotype D patients with HBeAg-negative chronic hepatitis B. Hepatology 52, 387A (2010).
Hadziyannis, S. et al. Outcome of HBeAg-negative chronic hepatitis B (CHG) 5 years after discontinuation of long term adefovir dipivoxil (ADV) treatment (abstract 18). J. Hepatol. 50, S9 (2009).
Yuen, M. F. et al. Factors preceding hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology 34, 785–791 (2001).
Keeffe, E. B. et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin. Gastroenterol. Hepatol. 6, 1315–1341 (2008).
Tenney, D. J. et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 49, 1503–1514 (2010).
Snow-Lampart, A. et al. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology doi: 10.1002/hep.24078.
Lampertico, P. et al. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 133, 1445–1451 (2007).
van Bommel, F. et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 51, 73–80 (2010).
Tan, J. et al. Tenofovir monotherapy is effective in hepatitis B patients with antiviral treatment failure to adefovir in the absence of adefovir-resistant mutations. J. Hepatol. 48, 391–398 (2008).
Author information
Authors and Affiliations
Contributions
H. Kwon contributed to the research, discussion and writing of this Review. A. S. Lok contributed to the research, discussion, writing and editing of this Review.
Corresponding author
Ethics declarations
Competing interests
Anna S. Lok receives grant/research support from and is a consultant for Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Roche/Genentech and Schering/Merck. She receives grant/research support from Innogenetics, and acts as a consultant for Abbot and Bayer. H. Kwon declares no competing interests.
Rights and permissions
About this article
Cite this article
Kwon, H., Lok, A. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 8, 275–284 (2011). https://doi.org/10.1038/nrgastro.2011.33
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.33
This article is cited by
-
Safety of Biologic Therapies in Patients with Moderate-to-Severe Plaque Psoriasis and Concomitant Viral Hepatitis: A Monocentric Retrospective Study
Dermatology and Therapy (2022)
-
Repurposing of Antazoline Hydrochloride as an Inhibitor of Hepatitis B Virus DNA Secretion
Virologica Sinica (2021)
-
In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol
Infectious Agents and Cancer (2020)
-
Serum hepatitis B core-related antigen predicts hepatocellular carcinoma in hepatitis B e antigen-negative patients
Journal of Gastroenterology (2020)
-
Management of Virologic Failure in Patients with Chronic Hepatitis B Treated with Nucleos(t)ide Analogues
Current Hepatology Reports (2019)