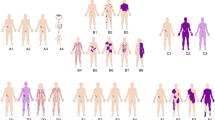
Key Points
-
Human cytogenetics was launched 46 years ago with the discovery that human somatic cells contain 46 chromosomes.
-
In the ensuing years, technological advances that have combined innovations in molecular biology, chemistry and instrumentation have repeatedly transformed human cytogenetics.
-
The resolution and sensitivity of cytogenetic analysis have improved more than 10,000-fold in an astoundingly short time — first, using banding technology, and later, using fluorescence in situ hybridization (FISH) — such that subtle alterations in chromosome composition can now be detected and analysed for their association with disease.
-
Using multicolour chromosome painting techniques, such as spectral karyotyping (SKY) and multicolour (M)-FISH, each chromosome can now be recognized easily by colour-coded labels, even in the highly rearranged karyotypes of tumour cells.
-
The karyotype of non-dividing cells can be analysed using FISH and comparative genome hybridization (CGH).
-
Other approaches, such as flow karyotyping, yield quantitative information on chromosomal content and structure and/or allow cytogeneticists to isolate specific chromosomes for molecular analyses.
-
Array-CGH, the most recently developed technology, allows genome-wide screens for the loss or gain of chromosomal material to be conducted at unprecedented resolution.
-
Most importantly, the cytogenetic map is cross-referenced to the human draft sequence at thousands of points. These connections greatly facilitate the translation of microscopically visible clues of the molecular basis of disease to the actual genes that are disrupted or altered in dosage.
Abstract
Human cytogenetics was born in 1956 with the fundamental, but empowering, discovery that normal human cells contain 46 chromosomes. Since then, this field and our understanding of the link between chromosomal defects and disease have grown in spurts that have been fuelled by advances in cytogenetic technology. As a mature enterprise, cytogenetics now informs human genomics, disease and cancer genetics, chromosome evolution and the relationship of nuclear structure to function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tjio, H. J. & Levan, A. The chromosome numbers of man. Hereditas 42, 1–6 (1956).This paper provides the first correct count of human chromosome number, which was independently confirmed by Ford and Hamerton (reference 3 ) in the same year.
Hsu, T. C. Human and Mammalian Cytogenetics: an Historical Perspective (Springer, New York, 1979).
Ford, C. E. & Hamerton, J. L. The chromosomes of man. Nature 178, 1020–1023 (1956).
Painter, T. S. Studies in mammalian spermatogenesis. II. The spermatogenesis of man. J. Exp. Zool. 37, 291–321 (1923).
Watson, J. R. D. & Crick, F. H. C. A structure for deoxyribose nucleic acid. Nature 171, 737–738 (1953).
Vogel, F. & Motulsky, A. G. Human Genetics: Problems and Approaches (Springer, Berlin, 1997).
Chicago Conference 1966. Standardization in Human Cytogenetics. Birth Defects: Original Article Series Vol. 2, No. 2 (The National Foundation, New York, 1966).
Lejeune, J., Gautier, M. & Turpin, M. R. Etude des chromosomes somatiques de neuf enfants mongoliens. C. R. Acad. Sci. (Paris) 248, 1721–1722 (1959).
Ford, C. E., Miller, O. J., Polani, P. E., de Almeida, J. C. & Briggs, J. H. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome). Lancet 1, 711–713 (1959).
Jacobs, P. A. & Strong, J. A. A case of human intersexuality having a possible XXY sex-determininig mechanism. Nature 183, 302–303 (1959).
Clendenin, T. M. & Bernirschke, K. Chromosome studies on spontaneous abortions. Lab. Invest. 12, 1281–1292 (1963).
Nowell, P. C. & Hungerford, D. A. A minute chromosome in human chronic granulocytic leukemia. Science 132, 1497–1501 (1960).
Rowley, J. D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293 (1973).
Heisterkamp, N., Stam, K., Groffen, J., de Klein, A. & Grosveld, G. Structural organization of the bcr gene and its role in the Ph′ translocation. Nature 315, 758–761 (1985).
Deininger, M. W., Goldman, J. M. & Melo, J. V. The molecular biology of chronic myeloid leukemia. Blood 96, 3343–3356 (2000).
Druker, B. J. Perspectives on the development of a molecularly targeted agent. Cancer Cell 1, 31–36 (2002).
Lejeune, J. et al. Trois cas de deletion partielle du bras court d'un chromosome 5. C. R. Acad. Sci. (Paris) 257, 3098–3102 (1963).
Lele, K. P., Penrose, L. S. & Stallard, H. B. Chromosome deletion in a case of retinoblastoma. Ann. Hum. Genet. 27, 171–174 (1963).
Cavenee, W. K. et al. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 305, 779–784 (1983).
Knudson, A. G. Jr. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl Acad. Sci. USA 68, 820–823 (1971).
Donahue, R. P., Bias, W. B., Renwick, J. H. & McKusick, V. A. Probable assignment of the Duffy blood group locus to chromosome 1 in man. Proc. Natl Acad. Sci. USA 61, 949–955 (1968).
Caspersson, T. et al. Chemical differentiation along metaphase chromosomes. Exp. Cell Res. 49, 219–222 (1968).This paper introduces the technique for identifying chromosomes by their banding pattern, a revolutionary step in human cytogenetics.
Yunis, J. J. Mid-prophase human chromosomes. The attainment of 2000 bands. Hum. Genet. 56, 293–298 (1981).
Paris Conference 1971. Standardization in Human Cytogenetics. Birth Defects: Original Article Series Vol. 8, No. 7 (The National Foundation, New York, 1972); also in Cytogenetics 11, 313–362 (1972).
Harris, H. & Watkins, J. F. Hybrid cells from mouse and man: artificial heterokaryons of mammalian cells from different species. Nature 205, 640–646 (1965).
Ephrussi, B. & Weiss, M. C. Interspecific hybridization of somatic cells. Proc. Natl Acad. Sci. USA 53, 1040–1042 (1965).
Weiss, M. C. & Green, H. Human–mouse hybrid cell lines containing partial complements of human chromosomes and functioning human genes. Proc. Natl Acad. Sci. USA 58, 1104–1111 (1967).
Ruddle, F. H. et al. Linkage relationships of seventeen human gene loci as determined by man–mouse somatic cell hybrids. Nature New Biol. 232, 69–73 (1971).
Budarf, M. L. et al. Regional localization of over 300 loci on human chromosome 22 using a somatic cell hybrid mapping panel. Genomics 35, 275–288 (1996).
Cox, D. R., Burmeister, M., Price, E. R., Kim, S. & Myers, R. M. Radiation hybrid mapping: a somatic cell genetic method for constructing high-resolution maps of mammalian chromosomes. Science 250, 245–250 (1990).
Carrano, A. V., Gray, J. W., Langlois, R. G., Burkhart-Schultz, K. J. & Van Dilla, M. A. Measurement and purification of human chromosomes by flow cytometry and sorting. Proc. Natl Acad. Sci. USA 76, 1382–1384 (1979).
Langlois, R. G., Yu, L. C., Gray, J. W. & Carrano, A. V. Quantitative karyotyping of human chromosomes by dual beam flow cytometry. Proc. Natl Acad. Sci. USA 79, 7876–7880 (1982).
Trask, B., van den Engh, G., Mayall, B. & Gray, J. W. Chromosome heteromorphism quantified by high-resolution bivariate flow karyotyping. Am. J. Hum. Genet. 45, 739–752 (1989).
Trask, B., van den Engh, G., Nussbaum, R., Schwartz, C. & Gray, J. Quantification of the DNA content of structurally abnormal X chromosomes and X chromosome aneuploidy using high resolution bivariate flow karyotyping. Cytometry 11, 184–195 (1990).
Lebo, R. V. Chromosome sorting and DNA sequence localization. Cytometry 3, 145–154 (1982).
Krumlauf, R., Jeanpierre, M. & Young, B. D. Construction and characterization of genomic libraries from specific human chromosomes. Proc. Natl Acad. Sci. USA 79, 2971–2975 (1982).
Van Dilla, M. A. & Deaven, L. L. Construction of gene libraries for each human chromosome. Cytometry 11, 208–218 (1990).
Telenius, H. et al. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosomes Cancer 4, 257–263 (1992).
Mefford, H. C., Linardopoulou, E., Coil, D., van den Engh, G. & Trask, B. J. Comparative sequencing of a multicopy subtelomeric region containing olfactory receptor genes reveals multiple interactions between non-homologous chromosomes. Hum. Mol. Genet. 10, 2363–2372 (2001).
John, H. A., Birnstiel, M. L. & Jones, K. W. RNA–DNA hybrids at the cytological level. Nature 223, 582–587 (1969).
Pardue, M. L. & Gall, J. G. Molecular hybridization of radioactive DNA to the DNA of cytological preparations. Proc. Natl Acad. Sci. USA 64, 600–604 (1969).
Langer-Safer, P. R., Levine, M. & Ward, D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc. Natl Acad. Sci. USA 79, 4381–4385 (1982).
Van Prooijen-Knegt, A. C. et al. In situ hybridization of DNA sequences in human metaphase chromosomes visualized by an indirect fluorescent immunocytochemical procedure. Exp. Cell Res. 141, 397–407 (1982).
Landegent, J. E. et al. Chromosomal localization of a unique gene by non-autoradiographic in situ hybridization. Nature 317, 175–177 (1985).This report shows, for the first time, the localization of a human gene to chromosome bands by non-isotopic techniques.
Korenberg, J. R., Chen, X. N., Adams, M. D. & Venter, J. C. Toward a cDNA map of the human genome. Genomics 29, 364–370 (1995).
Landegent, J. E., Jansen in de Wal, N., Dirks, R. W., Baao, F. & van der Ploeg, M. Use of whole cosmid cloned genomic sequences for chromosomal localization by non-radioactive in situ hybridization. Hum. Genet. 77, 366–370 (1987).
Lansdorp, P. M. et al. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 5, 685–691 (1996).
Meyne, J. & Goodwin, E. H. Direction of DNA sequences within chromatids determined using strand-specific FISH. Chromosome Res. 3, 375–378 (1995).
Bailey, S. M., Meyne, J., Cornforth, M. N., McConnell, T. S. & Goodwin, E. H. A new method for detecting pericentric inversions using COD-FISH. Cytogenet. Cell Genet. 75, 248–253 (1996).
Cornforth, M. N. & Eberle, R. L. Termini of human chromosomes display elevated rates of mitotic recombination. Mutagenesis 16, 85–89 (2001).
Trask, B. J. Fluorescence in situ hybridization: applications in cytogenetics and gene mapping. Trends Genet. 7, 149–154 (1991).
Lichter, P. et al. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science 247, 64–69 (1990).
BAC Resource Consortium. Integration of cytogenetic landmarks into the draft sequence of the human genome. Nature 409, 953–958 (2001).This paper reports the assembly and application of more than 8,000 FISH-mapped, sequence-tagged BACs, which tightly integrate the cytogenetic and sequence maps in the human genome.
Liu, P. et al. Fusion between transcription factor CBFβ/PEBP2β and a myosin heavy chain in acute myeloid leukemia. Science 261, 1041–1044 (1993).
Kundu, M. & Liu, P. P. Function of the inv(16) fusion gene CBFB–MYH11. Curr. Opin. Hematol. 8, 201–205 (2001).
Knoll, J. H. et al. Angelman and Prader–Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am. J. Med. Genet. 32, 285–290 (1989).
Lalande, M. Parental imprinting and human disease. Annu. Rev. Genet. 30, 173–195 (1996).
Stankiewicz, P. & Lupski, J. R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 18, 74–82 (2002).
Cremer, T. et al. Detection of chromosome aberrations in the human interphase nucleus by visualization of specific target DNAs with radioactive and non-radioactive in situ hybridization techniques: diagnosis of trisomy 18 with probe L1.84. Hum. Genet. 74, 346–352 (1986).
Pinkel, D., Straume, T. & Gray, J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc. Natl Acad. Sci. USA 83, 2934–2938 (1986).
Lupski, J. R. et al. DNA duplication associated with Charcot–Marie–Tooth disease type 1A. Cell 66, 219–232 (1991).
Tkachuk, D. C. et al. Detection of bcr–abl fusion in chronic myelogeneous leukemia by in situ hybridization. Science 250, 559–562 (1990).This paper reports the identification of the Philadelphia chromosome in interphase nuclei using two-colour FISH.
Selig, S., Okumura, K., Ward, D. C. & Cedar, H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 11, 1217–1225 (1992).
Kitsberg, D. et al. Allele-specific replication timing of imprinted gene regions. Nature 364, 459–463 (1993).
van den Engh, G., Sachs, R. & Trask, B. J. Estimating genomic distance from DNA sequence location in cell nuclei by a random walk model. Science 257, 1410–1412 (1992).
Wiegant, J. et al. High-resolution in situ hybridization using DNA halo preparations. Hum. Mol. Genet. 1, 587–591 (1992).
Parra, I. & Windle, B. High resolution visual mapping of stretched DNA by fluorescent hybridization. Nature Genet. 5, 17–21 (1993).
Kuwano, A., Ledbetter, S. A., Dobyns, W. B., Emanuel, B. S. & Ledbetter, D. H. Detection of deletions and cryptic translocations in Miller–Dieker syndrome by in situ hybridization. Am. J. Hum. Genet. 49, 707–714 (1991).
Schrock, E. et al. Multicolor spectral karyotyping of human chromosomes. Science 273, 494–497 (1996).This paper and reference 70 show how each human chromosome can be painted with one of 24 colours for automated karyotype analysis.
Speicher, M. R., Gwyn Ballard, S. & Ward, D. C. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nature Genet. 12, 368–375 (1996).
Lichter, P. et al. Rapid detection of human chromosome 21 aberrations by in situ hybridization. Proc. Natl Acad. Sci. USA 85, 9664–9668 (1988).
Pinkel, D. et al. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc. Natl Acad. Sci. USA 85, 9138–9142 (1988).
Vooijs, M. et al. Libraries for each human chromosome, constructed from sorter-enriched chromosomes by using linker–adaptor PCR. Am. J. Hum. Genet. 52, 586–597 (1993).
Meltzer, P. S., Guan, X. Y., Burgess, A. & Trent, J. M. Rapid generation of region specific probes by chromosome microdissection and their application. Nature Genet. 1, 24–28 (1992).
Ried, T., Landes, G., Dackowski, W., Klinger, K. & Ward, D. C. Multicolor fluorescence in situ hybridization for the simultaneous detection of probe sets for chromosomes 13, 18, 21, X and Y in uncultured amniotic fluid cells. Hum. Mol. Genet. 1, 307–313 (1992).
Mrozek, K., Heinonen, K., Theil, K. S. & Bloomfield, C. D. Spectral karyotyping in patients with acute myeloid leukemia and a complex karyotype shows hidden aberrations, including recurrent overrepresentation of 21q, 11q, and 22q. Genes Chromosomes Cancer 34, 137–153 (2002).
Liyanage, M. et al. Multicolour spectral karyotyping of mouse chromosomes. Nature Genet. 14, 312–315 (1996).
Loucas, B. D. & Cornforth, M. N. Complex chromosome exchanges induced by γ-rays in human lymphocytes: an mFISH study. Radiat. Res. 155, 660–671 (2001).
Sachs, R. K., Hlatky, L. R. & Trask, B. J. Radiation-produced chromosome aberrations: colourful clues. Trends Genet. 16, 143–146 (2000).
Knight, S. J. et al. An optimized set of human telomere clones for studying telomere integrity and architecture. Am. J. Hum. Genet. 67, 320–332 (2000).
Flint, J. et al. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nature Genet. 9, 132–140 (1995).
Jauch, A. et al. Reconstruction of genomic rearrangements in great apes and gibbons by chromosome painting. Proc. Natl Acad. Sci. USA 89, 8611–8615 (1992).
Weinberg, J. & Stanyon, R. Comparative painting of mammalian chromosomes. Curr. Opin. Genet. Dev. 7, 784–791 (1997).A review of the chromosomal rearrangements that have occurred during evolution as detected by cross-species FISH using chromosome-specific paints and locus-specific probes.
Stanyon, R. et al. Reciprocal chromosome painting shows that genomic rearrangement between rat and mouse proceeds ten times faster than between humans and cats. Cytogenet. Cell. Genet. 84, 150–155 (1999).
Ostrander, E. A. & Kruglyak, L. Unleashing the canine genome. Genome Res. 10, 1271–1274 (2000).
Breen, M., Thomas, R., Binns, M. M., Carter, N. P. & Langford, C. F. Reciprocal chromosome painting reveals detailed regions of conserved synteny between the karyotypes of the domestic dog (Canis familiaris) and human. Genomics 61, 145–155 (1999).
Kallioniemi, A. et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 258, 818–821 (1992).The first paper to describe CGH, which makes it possible to detect loss and gain of chromosomal material in non-dividing tumour cells.
Shayesteh, L. et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nature Genet. 21, 99–102 (1999).
Klein, C. A. et al. Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc. Natl Acad. Sci. USA 96, 4494–4499 (1999).
Pinkel, D. et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nature Genet. 20, 207–211 (1998).Provides the first proof-of-principle demonstration of array-CGH using BAC clones that were selected to mark specific points along the genome as hybridization targets.
Schriml, L. M. et al. Tyramide signal amplification (TSA)-FISH applied to mapping PCR-labeled probes less than 1 kb in size. Biotechniques 27, 608–613 (1999).
Zhong, X. B., Lizardi, P. M., Huang, X. H., Bray-Ward, P. L. & Ward, D. C. Visualization of oligonucleotide probes and point mutations in interphase nuclei and DNA fibers using rolling circle DNA amplification. Proc. Natl Acad. Sci. USA 98, 3940–3945 (2001).
Suzuki, H. et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nature Genet. 31, 141–149 (2002).
Iyer, V. R. et al. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409, 533–538 (2001).
Gray, J. W. et al. Flow karyotyping and sorting of human chromosomes. Cold Spring Harbor Symp. Quant. Biol. 51, 141–149 (1986).
Zitzelsberger, H. F., O'Brien, B. & Weier, H. U. G. in FISH Technology (eds Rautenstrauss, B. & Liehr, T.) 408–424 (Springer, Heidelberg, 2002).
McNeil, N. & Ried, T. Novel molecular cytogenetic techniques for identifying complex chromosomal rearrangements: technology and applications in molecular medicine. Expert Rev. Mol. Med. [online] 14 September 2000 〈http://www-ermm.cbcu.cam.ac.uk/00001940h.htm〉 (2000).
Padilla-Nash, H. M. et al. Molecular cytogenetic analysis of the bladder carcinoma cell line BK-10 by spectral karyotyping. Genes Chromosomes Cancer 25, 53–59 (1999).
Acknowledgements
I would like to thank the National Institutes of Health, US Department of Energy and the Fred Hutchinson Cancer Research Center for their present and past support. B.J.T. has significant financial interest in Cytopeia and Dako Cytomation, which are companies that develop and market flow cytometers.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
OMIM
FURTHER INFORMATION
Atlas of Genetics and Cytogenetics in Oncology and Haematology
Cytogenetic Forum at Waisman Center, University of Wisconsin
Glossary
- KNUDSON'S TWO-HIT MODEL
-
First proposed by Alfred Knudson in 1971, this model indicates that successive hits, such as deletion or mutation, in both alleles of a tumour-suppressor gene are required to turn a normal cell into a cancer cell.
- DUFFY BLOOD GROUP
-
An antigenic variant of a chemokine receptor that is expressed on red blood cells.
- AMNIOCENTESIS
-
A procedure in which a small sample of amniotic fluid is drawn out of the uterus through a needle inserted into the abdomen. The fluid is then analysed to detect genetic abnormalities in the fetus or to determine the sex of the fetus.
- G-BANDS/R-BANDS
-
Chromosome banding pattern produced by Giemsa staining (G-bands); the reciprocal pattern (reverse or R-bands) can be produced with various other staining procedures.
- PEPTIDE NUCLEIC ACID
-
(PNA). An analogue of DNA in which the backbone is a pseudopeptide rather than a sugar. PNA mimics the behaviour of DNA, but, because PNA has a neutral backbone, it binds complementary nucleic-acid strands more strongly and with greater specificity than an oligonucleotide.
- COD-FISH
-
(Chromosome orientation and direction-fluorescence in situ hybridization). In this technique, single-stranded probes hybridize to one chromatid of a metaphase chromosome, because the most recently synthesized strand in each chromatid is specifically degraded before hybridization. A probe that recognizes the cytosine-rich strand of the telomeric repeat provides orientation by marking the 5′-end of each chromatid.
- BAC, PAC AND YAC
-
Cloning vector system able to accomodate large genomic fragments. BACs and PACs are grown in bacteria; YACs are grown in yeast.
- PERICENTRIC INVERSION
-
A structural alteration to a chromosome that results from breakage, inversion and reinsertion of a fragment that spans the centromere.
- IMPRINTING
-
A genetic mechanism by which genes are selectively expressed from the maternal or paternal homologue of a chromosome.
- CHARCOT–MARIE–TOOTH SYNDROME
-
An inherited degenerative peripheral nerve disorder that causes progressive muscle weakness and atrophy in the feet, legs, hands and forearms.
- SMITH–MAGENIS SYNDROME
-
A rare condition that is associated with developmental delay, characteristic facial and other anatomical abnormalities, learning difficulties and behavioural problems, such as the tendency to harm oneself.
- DEGENERATE OLIGONUCLEOTIDE-PRIMED (DOP) PCR/LINKER–ADAPTOR PCR
-
DOP-PCR uses partially degenerate primers to amplify sequences at dispersed sites in a sample. In linker-adaptor PCR, the DNA sample is digested with a restriction enzyme, the ends are ligated to an adaptor oligonucleotide, and the ligated fragments are amplified using PCR primers that are complementary to the linker-adaptor oligonucleotide. Both techniques generate large pools of fragments that almost completely represent the starting sample.
- INTERFEROMETER
-
A device that uses an interference pattern to determine wave frequency, length or velocity.
- TYRAMIDE CHEMISTRY
-
A labelling system that uses a hybridization probe that is directly or indirectly labelled with peroxidase. The peroxidase catalyses the localized deposition of a reactive tyramide-labelled tag (for example, biotin or fluorescent dyes).
Rights and permissions
About this article
Cite this article
Trask, B. Human cytogenetics: 46 chromosomes, 46 years and counting. Nat Rev Genet 3, 769–778 (2002). https://doi.org/10.1038/nrg905
Issue Date:
DOI: https://doi.org/10.1038/nrg905
This article is cited by
-
The reckoning of chromosomal instability: past, present, future
Chromosome Research (2024)
-
Accuracy and depth evaluation of clinical low pass genome sequencing in the detection of mosaic aneuploidies and CNVs
BMC Medical Genomics (2023)
-
Ultrastructural visualization of 3D chromatin folding using volume electron microscopy and DNA in situ hybridization
Nature Communications (2020)
-
A comprehensive review of the Multidisciplinarity in Karyotypization
Health and Technology (2020)
-
Molecular cytogenetics and its application to major flowering ornamental crops
Horticulture, Environment, and Biotechnology (2020)