Key Points
-
tRNAs are key molecules for translation that deliver amino acids to the ribosome to translate genetic information in an mRNA template-directed manner. However, emerging evidence suggests that tRNAs have a more central role in a stress response paradigm by functioning directly as signalling molecules in adaptive translation.
-
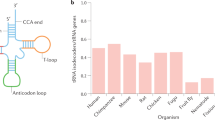
tRNA composition differs markedly in various cells and tissues to meet diverse translational demands. The expression and abundance of tRNA pools are shaped by tissue-specific chromatin accessibility and RNA polymerase III interactions.
-
Stress-related tRNA functions operate on different timescales and include reprogramming covalent modifications to modulate decoding fidelity, fragmentation in the anticodon loop to interfere with translation initiation, and global tRNA depletion by deactivation of the ubiquitous CCA termini.
-
Mutations in tRNAs and in tRNA processing or modifying genes are linked to several human diseases, with some tissues affected more than others. Mitochondria generally carry a single copy of each tRNA-encoding gene and are particularly vulnerable to deleterious mutations.
-
Many human diseases (for example, cancer-related and neurodegenerative pathologies) do not have a direct mutational link to tRNAs but alter tRNA pools as a secondary effect of the disease biology.
-
A complete inventory of the tRNA pool (and tRNA fragments) in each tissue is necessary to understand tissue-specific features that modulate pathology.
Abstract
tRNAs, nexus molecules between mRNAs and proteins, have a central role in translation. Recent discoveries have revealed unprecedented complexity of tRNA biosynthesis, modification patterns, regulation and function. In this Review, we present emerging concepts regarding how tRNA abundance is dynamically regulated and how tRNAs (and their nucleolytic fragments) are centrally involved in stress signalling and adaptive translation, operating across a wide range of timescales. Mutations in tRNAs or in genes affecting tRNA biogenesis are also linked to complex human diseases with surprising heterogeneity in tissue vulnerability, and we highlight cell-specific aspects that modulate the disease penetrance of tRNA-based pathologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rodnina, M. V. & Wintermeyer, W. The ribosome as a molecular machine: the mechanism of tRNA–mRNA movement in translocation. Biochem. Soc. Trans. 39, 658–662 (2011).
Giege, R. Toward a more complete view of tRNA biology. Nature Struct. Mol. Biol. 15, 1007–1014 (2008).
Gebetsberger, J. & Polacek, N. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 10, 1798–1806 (2013).
Thompson, D. M. & Parker, R. Stressing out over tRNA cleavage. Cell 138, 215–219 (2009).
Abbott, J. A., Francklyn, C. S. & Robey-Bond, S. M. Transfer RNA and human disease. Front. Genet. 5, 158 (2014).
Blanco, S. & Frye, M. Role of RNA methyltransferases in tissue renewal and pathology. Curr. Opin. Cell Biol. 31C, 1–7 (2014).
Schon, E. A., DiMauro, S. & Hirano, M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nature Rev. Genet. 13, 878–890 (2012). This review highlights crucial aspects of somatic mutations in some human pathologies.
Suzuki, T., Nagao, A. & Suzuki, T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 45, 299–329 (2011).
Yao, P. & Fox, P. L. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol. Med. 5, 332–343 (2013).
Durdevic, Z. & Schaefer, M. tRNA modifications: necessary for correct tRNA-derived fragments during the recovery from stress? Bioessays 35, 323–327 (2013).
El Yacoubi, B., Bailly, M. & de Crecy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 46, 69–95 (2012).
Gustilo, E. M., Vendeix, F. A. & Agris, P. F. tRNA's modifications bring order to gene expression. Curr. Opin. Microbiol. 11, 134–140 (2008).
Phizicky, E. M. & Hopper, A. K. tRNA biology charges to the front. Genes Dev. 24, 1832–1860 (2010).
Ibba, M. & Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000).
Ramakrishnan, V. Ribosome structure and the mechanism of translation. Cell 108, 557–572 (2002).
Lang, B. F., Gray, M. W. & Burger, G. Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 33, 351–397 (1999).
Ambrogelly, A., Palioura, S. & Soll, D. Natural expansion of the genetic code. Nature Chem. Biol. 3, 29–35 (2007).
Chan, P. P. & Lowe, T. M. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 37, D93–D97 (2009).
Iben, J. R. & Maraia, R. J. tRNA gene copy number variation in humans. Gene 536, 376–384 (2014).
Parisien, M., Wang, X. & Pan, T. Diversity of human tRNA genes from the 1000-genomes project. RNA Biol. 10, 1853–1867 (2013).
Goodenbour, J. M. & Pan, T. Diversity of tRNA genes in eukaryotes. Nucleic Acids Res. 34, 6137–6146 (2006).
Kutter, C. et al. Pol III binding in six mammals shows conservation among amino acid isotypes despite divergence among tRNA genes. Nature Genet. 43, 948–955 (2011).
Thompson, M., Haeusler, R. A., Good, P. D. & Engelke, D. R. Nucleolar clustering of dispersed tRNA genes. Science 302, 1399–1401 (2003).
Dong, H., Nilsson, L. & Kurland, C. G. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 260, 649–663 (1996).
Zhang, G., Lukoszek, R., Mueller-Roeber, B. & Ignatova, Z. Different sequence signatures in the upstream regions of plant and animal tRNA genes shape distinct modes of regulation. Nucleic Acids Res. 39, 3331–3339 (2011).
Ouyang, C., Martinez, M. J., Young, L. S. & Sprague, K. U. TATA-binding protein–TATA interaction is a key determinant of differential transcription of silkworm constitutive and silk gland-specific tRNA(Ala) genes. Mol. Cell. Biol. 20, 1329–1343 (2000).
Fredrick, K. & Ibba, M. How the sequence of a gene can tune its translation. Cell 141, 227–229 (2010).
Zhang, G. & Ignatova, Z. Folding at the birth of the nascent chain: coordinating translation with co-translational folding. Curr. Opin. Struct. Biol. 21, 25–31 (2011).
Sauna, Z. E. & Kimchi-Sarfaty, C. Understanding the contribution of synonymous mutations to human disease. Nature Rev. Genet. 12, 683–691 (2011).
Zhou, M. et al. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature 495, 111–115 (2013).
Novoa, E. M. & Ribas de Pouplana, L. Speeding with control: codon usage, tRNAs, and ribosomes. Trends Genet. 28, 574–581 (2012).
Plotkin, J. B. & Kudla, G. Synonymous but not the same: the causes and consequences of codon bias. Nature Rev. Genet. 12, 32–42 (2011).
Novoa, E. M., Pavon-Eternod, M., Pan, T. & Ribas de Pouplana, L. A role for tRNA modifications in genome structure and codon usage. Cell 149, 202–213 (2012).
Dittmar, K. A., Goodenbour, J. M. & Pan, T. Tissue-specific differences in human transfer RNA expression. PLoS Genet. 2, e221 (2006). This paper presents quantitative assessment of tRNA abundance and reports broad variations in the tissue-specific expression of tRNA species.
Gingold, H. et al. A dual program for translation regulation in cellular proliferation and differentiation. Cell 158, 1281–1292 (2014).
Plotkin, J. B., Robins, H. & Levine, A. J. Tissue-specific codon usage and the expression of human genes. Proc. Natl Acad. Sci. USA 101, 12588–12591 (2004).
Lampson, B. L. et al. Rare codons regulate KRAS oncogenesis. Curr. Biol. 23, 70–75 (2013).
Barski, A. et al. Pol II and its associated epigenetic marks are present at Pol III-transcribed noncoding RNA genes. Nature Struct. Mol. Biol. 17, 629–634 (2010).
Oler, A. J. et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nature Struct. Mol. Biol. 17, 620–628 (2010).
McFarlane, R. J. & Whitehall, S. K. tRNA genes in eukaryotic genome organization and reorganization. Cell Cycle 8, 3102–3106 (2009).
Ledoux, S., Olejniczak, M. & Uhlenbeck, O. C. A sequence element that tunes Escherichia coli tRNAAlaGGC to ensure accurate decoding. Nature Struct. Mol. Biol. 16, 359–364 (2009).
Wohlgemuth, I., Pohl, C., Mittelstaet, J., Konevega, A. L. & Rodnina, M. V. Evolutionary optimization of speed and accuracy of decoding on the ribosome. Phil. Trans. R. Soc. B 366, 2979–2986 (2011).
Fedyunin, I. et al. tRNA concentration fine tunes protein solubility. FEBS Lett. 586, 3336–3340 (2012).
Bloom-Ackermann, Z. et al. A comprehensive tRNA deletion library unravels the genetic architecture of the tRNA pool. PLoS Genet. 10, e1004084 (2014).
Yona, A. H. et al. tRNA genes rapidly change in evolution to meet novel translational demands. Elife 2, e01339 (2013).
Geslain, R. & Pan, T. Functional analysis of human tRNA isodecoders. J. Mol. Biol. 396, 821–831 (2010).
de Nadal, E., Ammerer, G. & Posas, F. Controlling gene expression in response to stress. Nature Rev. Genet. 12, 833–845 (2011).
Levitz, R. et al. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 9, 1383–1389 (1990).
Haiser, H. J., Karginov, F. V., Hannon, G. J. & Elliot, M. A. Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic Acids Res. 36, 732–741 (2008).
Hsieh, L. C., Lin, S. I., Kuo, H. F. & Chiou, T. J. Abundance of tRNA-derived small RNAs in phosphate-starved Arabidopsis roots. Plant Signal Behav. 5, 537–539 (2010).
Jochl, C. et al. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res. 36, 2677–2689 (2008).
Thompson, D. M., Lu, C., Green, P. J. & Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14, 2095–2103 (2008).
Wang, Q. et al. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol. Ther. 21, 368–379 (2013).
Thompson, D. M. & Parker, R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell Biol. 185, 43–50 (2009).
Yamasaki, S., Ivanov, P., Hu, G. F. & Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 185, 35–42 (2009).
Saikia, M. et al. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J. Biol. Chem. 287, 42708–42725 (2012).
Czech, A., Wende, S., Morl, M., Pan, T. & Ignatova, Z. Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet. 9, e1003767 (2013).
Emara, M. M. et al. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 285, 10959–10968 (2010).
Ivanov, P., Emara, M. M., Villen, J., Gygi, S. P. & Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 43, 613–623 (2011). This paper reports the first observation that tRNA fragments specifically displace eIF4G and eIF4F initiation factors and inhibit protein initiation.
Zhang, Y. et al. Identification and characterization of an ancient class of small RNAs enriched in serum associating with active infection. J. Mol. Cell. Biol. 6, 172–174 (2014).
Durdevic, Z., Mobin, M. B., Hanna, K., Lyko, F. & Schaefer, M. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 4, 931–937 (2013).
Dhahbi, J. M. et al. 5′ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics 14, 298 (2013).
Holcik, M. & Sonenberg, N. Translational control in stress and apoptosis. Nature Rev. Mol. Cell Biol. 6, 318–327 (2005).
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013).
Whitney, M. L., Hurto, R. L., Shaheen, H. H. & Hopper, A. K. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol. Biol. Cell 18, 2678–2686 (2007).
Murguia, J. R. & Serrano, R. New functions of protein kinase Gcn2 in yeast and mammals. IUBMB Life 64, 971–974 (2012).
Qiu, H. et al. Defects in tRNA processing and nuclear export induce GCN4 translation independently of phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell. Biol. 20, 2505–2516 (2000).
Chan, C. T. et al. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 6, e1001247 (2010). This study describes a highly sensitive mass spectrometry-based approach to simultaneously detect tRNA modification in yeast.
Chan, C. T. et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nature Commun. 3, 937 (2012).
Zinshteyn, B. & Gilbert, W. V. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 9, e1003675 (2013). Using translatome-wide analysis, this study shows that stress-induced mismodification of wobble position U34 reduces global gene expression by activating GCN4-mediated stress response and not by altered codon–anticodon pairing.
Johansson, M. J., Esberg, A., Huang, B., Bjork, G. R. & Bystrom, A. S. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell. Biol. 28, 3301–3312 (2008).
Chen, C., Tuck, S. & Bystrom, A. S. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 5, e1000561 (2009).
Ingolia, N. T. Ribosome profiling: new views of translation, from single codons to genome scale. Nature Rev. Genet. 15, 205–213 (2014).
Miranda, I. et al. Candida albicans CUG mistranslation is a mechanism to create cell surface variation. MBio 4, e00285-13 (2013).
Wiltrout, E., Goodenbour, J. M., Frechin, M. & Pan, T. Misacylation of tRNA with methionine in Saccharomyces cerevisiae. Nucleic Acids Res. 40, 10494–10506 (2012).
Netzer, N. et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462, 522–526 (2009). This study describes a new mechanism that protects cells against oxidative stress by misincorporation of methionine through non-Met-tRNAs.
Jones, T. E., Alexander, R. W. & Pan, T. Misacylation of specific nonmethionyl tRNAs by a bacterial methionyl-tRNA synthetase. Proc. Natl Acad. Sci. USA 108, 6933–6938 (2011).
Bender, A., Hajieva, P. & Moosmann, B. Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria. Proc. Natl Acad. Sci. USA 105, 16496–16501 (2008).
Schneider, A. Mitochondrial tRNA import and its consequences for mitochondrial translation. Annu. Rev. Biochem. 80, 1033–1053 (2011).
Brandon, M. C. et al. MITOMAP: a human mitochondrial genome database — 2004 update. Nucleic Acids Res. 33, D611–D613 (2005).
Moraes, C. T. et al. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nature Genet. 4, 284–288 (1993).
Flierl, A., Reichmann, H. & Seibel, P. Pathophysiology of the MELAS 3243 transition mutation. J. Biol. Chem. 272, 27189–27196 (1997).
Suzuki, T., Wada, T., Saigo, K. & Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 21, 6581–6589 (2002).
Kirino, Y. et al. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl Acad. Sci. USA 101, 15070–15075 (2004).
Yasukawa, T., Suzuki, T., Ishii, N., Ohta, S. & Watanabe, K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J. 20, 4794–4802 (2001).
Hasegawa, H., Matsuoka, T., Goto, Y. & Nonaka, I. Cytochrome-C-oxidase activity is deficient in blood-vessels of patients with myoclonus epilepsy with ragged-red fibers. Acta Neuropathol. 85, 280–284 (1993).
Naini, A. et al. Hypocitrullinemia in patients with MELAS: an insight into the “MELAS paradox”. J. Neurol. Sci. 229–230, 187–193 (2005).
Wang, S. et al. Maternally inherited essential hypertension is associated with the novel 4263A>G mutation in the mitochondrial tRNAIle gene in a large Han Chinese family. Circ. Res. 108, 862–870 (2011).
Liang, M. Hypertension as a mitochondrial and metabolic disease. Kidney Int. 80, 15–16 (2011).
Liu, Y. et al. Mitochondrial transfer RNAMet 4435A>G mutation is associated with maternally inherited hypertension in a Chinese pedigree. Hypertension 53, 1083–1090 (2009).
Ishimura, R. et al. RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 345, 455–459 (2014). This study reports that a mutation in a nuclear-encoded tRNA gene, which is specifically expressed in the CNS, may itself be phenotypically silent but epistatically exacerbates the deleterious effect of the mutation in a partner of the ribosome recycling protein Pelota.
Karaca, E. et al. Human CLP1 mutations alter tRNA biogenesis, affecting both peripheral and central nervous system function. Cell 157, 636–650 (2014).
Schaffer, A. E. et al. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 157, 651–663 (2014). References 92 and 93, which were simultaneously published, reveal a new link between a mutation in CLP1 kinase, impairment in pre-tRNA processing and human pathology.
Hanada, T. et al. CLP1 links tRNA metabolism to progressive motor-neuron loss. Nature 495, 474–480 (2013).
Latour, P. et al. A major determinant for binding and aminoacylation of tRNAAla in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot–Marie–Tooth disease. Am. J. Hum. Genet. 86, 77–82 (2010).
Guo, M. et al. Paradox of mistranslation of serine for alanine caused by AlaRS recognition dilemma. Nature 462, 808–812 (2009).
Lee, J. W. et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 (2006).
Sleigh, J. N., Grice, S. J., Burgess, R. W., Talbot, K. & Cader, M. Z. Neuromuscular junction maturation defects precede impaired lower motor neuron connectivity in Charcot–Marie–Tooth type 2D mice. Hum. Mol. Genet. 23, 2639–2650 (2014).
Finsterer, J. Central nervous system manifestations of mitochondrial disorders. Acta Neurol. Scand. 114, 217–238 (2006).
Alazami, A. M. et al. Mutation in ADAT3, encoding adenosine deaminase acting on transfer RNA, causes intellectual disability and strabismus. J. Med. Genet. 50, 425–430 (2013).
Martinez, F. J. et al. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J. Med. Genet. 49, 380–385 (2012).
Rodriguez, V. et al. Chromosome 8 BAC array comparative genomic hybridization and expression analysis identify amplification and overexpression of TRMT12 in breast cancer. Genes Chromosomes Cancer 46, 694–707 (2007).
Blanco, S. et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 33, 2020–2039 (2014). This paper shows that a mutation in a tRNA-modifying enzyme leads to mismodification of tRNAs and enhances their susceptibility to angiogenin-mediated cleavage with an enhanced effect in neuronal tissues.
Darnell, J. C. Defects in translational regulation contributing to human cognitive and behavioral disease. Curr. Opin. Genet. Dev. 21, 465–473 (2011).
Wei, F. Y. et al. Deficit of tRNALys modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Invest. 121, 3598–3608 (2011).
Zhou, B. et al. Identification of a splicing variant that regulates type 2 diabetes risk factor CDKAL1 level by a coding-independent mechanism in human. Hum. Mol. Genet. 23, 4639–4650 (2014).
Wei, F. Y. & Tomizawa, K. Functional loss of Cdkal1, a novel tRNA modification enzyme, causes the development of type 2 diabetes. Endocr. J. 58, 819–825 (2011).
Stumpf, C. R. & Ruggero, D. The cancerous translation apparatus. Curr. Opin. Genet. Dev. 21, 474–483 (2011).
White, R. J. RNA polymerase III transcription and cancer. Oncogene 23, 3208–3216 (2004).
Pavon-Eternod, M. et al. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 37, 7268–7280 (2009).
Zhou, Y., Goodenbour, J. M., Godley, L. A., Wickrema, A. & Pan, T. High levels of tRNA abundance and alteration of tRNA charging by bortezomib in multiple myeloma. Biochem. Biophys. Res. Commun. 385, 160–164 (2009).
Girstmair, H. et al. Depletion of cognate charged transfer RNA causes translational frameshifting within the expanded CAG stretch in huntingtin. Cell Rep. 3, 148–159 (2013).
Landwehrmeyer, G. B. et al. Huntington's disease gene: regional and cellular expression in brain of normal and affected individuals. Ann. Neurol. 37, 218–230 (1995).
Krokowski, D. et al. A self-defeating anabolic program leads to β-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J. Biol. Chem. 288, 17202–17213 (2013).
Kim, H. & Kim, J. S. A guide to genome engineering with programmable nucleases. Nature Rev. Genet. 15, 321–334 (2014).
Puri, P. et al. Systematic identification of tRNAome and its dynamics in Lactococcus lactis. Mol. Microbiol. 93, 944–956 (2014).
Phizicky, E. M. & Alfonzo, J. D. Do all modifications benefit all tRNAs? FEBS Lett. 584, 265–271 (2010).
Suzuki, T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 42, 7346–7357 (2014).
Torres, A. G., Batlle, E. & Ribas de Pouplana, L. Role of tRNA modifications in human diseases. Trends Mol. Med. http://dx.doi.org/10.1016/j.molmed.2014.01.008 (2014).
Czerwoniec, A. et al. MODOMICS: a database of RNA modification pathways. 2008 update. Nucleic Acids Res. 37, D118–121 (2009).
Waldron, C. & Lacroute, F. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J. Bacteriol. 122, 855–865 (1975).
Persson, B. C., Gustafsson, C., Berg, D. E. & Bjork, G. R. The gene for a transfer-RNA modifying enzyme, M5u54-methyltransferase, is essential for viability in Escherichia-coli. Proc. Natl Acad. Sci. USA 89, 3995–3998 (1992).
Lee, T. & Feig, A. L. The RNA binding protein Hfq interacts specifically with tRNAs. RNA 14, 514–523 (2008).
Navarre, W. W. & Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63, 174–229 (1999).
Graciet, E. et al. Aminoacyl-transferases and the N-end rule pathway of prokaryotic/eukaryotic specificity in a human pathogen. Proc. Natl Acad. Sci. USA 103, 3078–3083 (2006).
Roy, H. & Ibba, M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl Acad. Sci. USA 105, 4667–4672 (2008).
Jahn, D., Verkamp, E. & Soll, D. Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends Biochem. Sci. 17, 215–218 (1992).
Karakozova, M. et al. Arginylation of β-actin regulates actin cytoskeleton and cell motility. Science 313, 192–196 (2006).
Hou, Y. M. & Yang, X. Regulation of cell death by transfer RNA. Antioxid. Redox Signal 19, 583–594 (2013).
Raab, J. R. et al. Human tRNA genes function as chromatin insulators. EMBO J. 31, 330–350 (2012).
Miller, S. B., Yildiz, F. Z., Lo, J. A., Wang, B. & D'Souza, V. M. A structure-based mechanism for tRNA and retroviral RNA remodelling during primer annealing. Nature 515, 591–595 (2014).
Ruggero, K. et al. Small noncoding RNAs in cells transformed by human T-cell leukemia virus type 1: a role for a tRNA fragment as a primer for reverse transcriptase. J. Virol. 88, 3612–3622 (2014).
Sheppard, K. et al. From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res. 36, 1813–1825 (2008).
Parisien, M. et al. Discovering RNA–protein interactome by using chemical context profiling of the RNA–protein interface. Cell Rep. 3, 1703–1713 (2013).
Rudinger-Thirion, J., Lescure, A., Paulus, C. & Frugier, M. Misfolded human tRNA isodecoder binds and neutralizes a 3′ UTR-embedded Alu element. Proc. Natl Acad. Sci. USA 108, E794–802 (2011).
Lund, E. & Dahlberg, J. E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science 282, 2082–2085 (1998).
Smits, P. et al. Functional consequences of mitochondrial tRNA Trp and tRNA Arg mutations causing combined OXPHOS defects. Eur. J. Hum. Genet. 18, 324–329 (2010).
Levinger, L., Morl, M. & Florentz, C. Mitochondrial tRNA 3′ end metabolism and human disease. Nucleic Acids Res. 32, 5430–5441 (2004).
Wittenhagen, L. M. & Kelley, S. O. Dimerization of a pathogenic human mitochondrial tRNA. Nature Struct. Biol. 9, 586–590 (2002).
Chomyn, A., Enriquez, J. A., Micol, V., Fernandez-Silva, P. & Attardi, G. The mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode syndrome-associated human mitochondrial tRNALeuUUR mutation causes aminoacylation deficiency and concomitant reduced association of mRNA with ribosomes. J. Biol. Chem. 275, 19198–19209 (2000).
Calvaruso, M. A. et al. New mitochondrial tRNA HIS mutation in a family with lactic acidosis and stroke-like episodes (MELAS). Mitochondrion 11, 778–782 (2011).
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Schaller, A. et al. Impairment of mitochondrial tRNAIle processing by a novel mutation associated with chronic progressive external ophthalmoplegia. Mitochondrion 11, 488–496 (2011).
Souilem, S. et al. A novel mitochondrial tRNAIle point mutation associated with chronic progressive external ophthalmoplegia and hyperCKemia. J. Neurol. Sci. 300, 187–190 (2011).
McFarland, R. et al. Multiple neonatal deaths due to a homoplasmic mitochondrial DNA mutation. Nature Genet. 30, 145–146 (2002).
Sacconi, S. et al. Complex neurologic syndrome associated with the G1606A mutation of mitochondrial DNA. Arch. Neurol. 59, 1013–1015 (2002).
Lynn, S. et al. Mitochondrial diabetes: investigation and identification of a novel mutation. Diabetes 47, 1800–1802 (1998).
Seneca, S. et al. A mitochondrial tRNA aspartate mutation causing isolated mitochondrial myopathy. Am. J. Med. Genet. A 137A, 170–175 (2005).
Jones, C. N., Jones, C. I., Graham, W. D., Agris, P. F. & Spremulli, L. L. A disease-causing point mutation in human mitochondrial tRNAMet rsults in tRNA misfolding leading to defects in translational initiation and elongation. J. Biol. Chem. 283, 34445–34456 (2008).
Tulinius, M. et al. Leigh syndrome with cytochrome-c oxidase deficiency and a single T insertion nt 5537 in the mitochondrial tRNATrp gene. Neuropediatrics 34, 87–91 (2003).
t Hart, L. M. et al. Evidence that the mitochondrial leucyl tRNA synthetase (LARS2) gene represents a novel type 2 diabetes susceptibility gene. Diabetes 54, 1892–1895 (2005).
Bonnefond, L. et al. Crystal structure of human mitochondrial tyrosyl-tRNA synthetase reveals common and idiosyncratic features. Structure 15, 1505–1516 (2007).
Scheper, G. C. et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nature Genet. 39, 534–539 (2007).
Pierce, S. B. et al. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl Acad. Sci. USA 108, 6543–6548 (2011).
Begley, U. et al. A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-α. EMBO Mol. Med. 5, 366–383 (2013).
Pavon-Eternod, M. et al. Vaccinia and influenza A viruses select rather than adjust tRNAs to optimize translation. Nucleic Acids Res. 41, 1914–1921 (2013).
Clarke, P., Leser, J. S., Bowen, R. A. & Tyler, K. L. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. MBio 5, e00902–e00914 (2014).
Pavon-Eternod, M., Wei, M., Pan, T. & Kleiman, L. Profiling non-lysyl tRNAs in HIV-1. RNA 16, 267–273 (2010).
Scheper, G. C., van der Knaap, M. S. & Proud, C. G. Translation matters: protein synthesis defects in inherited disease. Nature Rev. Genet. 8, 711–723 (2007).
Acknowledgements
The authors acknowledge careful reading of the manuscript and numerous suggestions by R. Smock and laboratory members. Work in the authors' laboratory on tRNA and translation is supported by grants from Deutsche Forschungsgemeinschaft, German Federal Ministry of Education and Research, and Marie–Curie Training Network of the European Union.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Anticodon
-
Nucleotides 34, 35 and 36 of each tRNA that recognize a specific codon of mRNA.
- A-site
-
The site of entry of the aminoacyl tRNA in the ribosome.
- Codon
-
Three consecutive nucleotides on mRNA that encode one amino acid.
- Translational frameshifting
-
A shift in the linear readthrough of mRNA in which the ribosome reads the second or the third nucleotide of a codon as the first nucleotide.
- Wobbling
-
Non-Watson–Crick base pairing between the third base in the codon with the first nucleotide of the tRNA anticodon (nucleotide 34 in tRNA numbering).
- tRNA isoacceptors
-
Different tRNA species carrying the same amino acids but with different anticodon sequences.
- tRNA isodecoders
-
Distinct tRNA species bearing the same amino acids and anticodons but with sequence variations in the tRNA body.
- Transcription factors
-
Proteins that bind to specific sequences in DNA and control the transcription of a gene.
- Synonymous substitutions
-
Substitutions of nucleotides in the exons of protein-coding genes that do not change the encoded amino acid.
- Codon bias
-
The difference in occurrence of codons encoding the same amino acid.
- Paralogous genes
-
Genes that arose from a duplication event but have diverged from a parent copy by mutation and selection drift; they may evolve new functions.
- Proteotoxic stress
-
A collective term to describe the intracellular stress caused by toxic protein aggregation.
- tRNA isoacceptor family
-
A family of all tRNA isoacceptors carrying the same amino acid.
- Endonuclease
-
An enzyme that hydrolyses the phosphodiester bond between two nucleotides in a sequence.
- RNA interference
-
A process by which short RNA sequences block gene expression by binding to specific mRNAs to cause their destruction.
- Homoplasmy
-
The presence of a single mitochondrial-encoded tDNA genotype in a cell.
- Heteroplasmy
-
The presence of a mixture of more than one mitochondrial-encoded tDNA genotype in a cell.
- tRNAome
-
The collective definition of the entire set of tRNAs expressed in a cell, a tissue or an organism at a given time.
Rights and permissions
About this article
Cite this article
Kirchner, S., Ignatova, Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 16, 98–112 (2015). https://doi.org/10.1038/nrg3861
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3861
This article is cited by
-
METTL1 mediated tRNA m7G modification promotes leukaemogenesis of AML via tRNA regulated translational control
Experimental Hematology & Oncology (2024)
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2024)
-
Novel structures and evolution of tRNA genes: insight into the chloroplast tRNAs of family Sapindaceae
Genetic Resources and Crop Evolution (2024)
-
Emerging roles of tRNA-derived fragments in cancer
Molecular Cancer (2023)
-
A novel tyrosine tRNA-derived fragment, tRFTyr, induces oncogenesis and lactate accumulation in LSCC by interacting with LDHA
Cellular & Molecular Biology Letters (2023)