Key Points
-
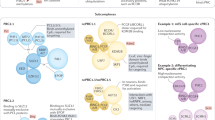
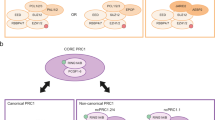
Many new Polycomb repressive complex 1 (PRC1) components have been identified in mammals and Drosophila melanogaster, which results in alternative complexes that are centred on the E3 ubiquitin-protein ligase RING2; not all of these alternative complexes include a chromodomain-containing Polycomb homologue.
-
Alternative PRC2 complexes have also been discovered in mammals and D. melanogaster.
-
Although much is still unclear, some of these variant complexes may act together. Others have tissue-specific roles in differentiation.
-
Histone H2A ubiquitylation and deubiquitylation are both important for Polycomb group-mediated gene silencing.
-
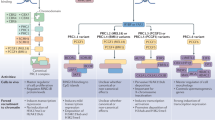
Mammalian systems reveal multiple strategies of recruitment of PRC1, PRC2 and their variant complexes, including the use of non-coding RNAs, Polycomb response elements and CpG islands.
-
Mutations that affect the methylation of histone H3 lysine 27 (H3K27), as well as mutations in H3K27 itself, reveal surprising effects on both the kinetics of PRC2 function and its role in disease.
-
Enhancer of zeste homologue 2 can methylate non-histone proteins with both activating and repressing effects, which indicates its roles beyond chromatin.
Abstract
Polycomb group (PcG) proteins are epigenetic repressors that are essential for the transcriptional control of cell differentiation and development. PcG-mediated repression is associated with specific post-translational histone modifications and is thought to involve both biochemical and physical modulation of chromatin structure. Recent advances show that PcG complexes comprise a multiplicity of variants and are far more biochemically diverse than previously thought. The importance of these new PcG complexes for normal development and disease, their targeting mechanisms and their shifting roles in the course of differentiation are now the subject of investigation and the focus of this Review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jürgens, G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 316, 153–155 (1985).
Gaytán de Ayala Alonso, A. et al. Genetic screen identifies novel Polycomb group genes in Drosophila. Genetics 176, 2099–2108 (2007).
Poux, S., Kostic, C. & Pirrotta, V. Hunchback-independent silencing of late Ubx enhancers by a Polycomb group response element. EMBO J. 15, 4713–4722 (1996).
Leeb, M. et al. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 24, 265–276 (2010).
Zhou, W. et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol. Cell 29, 69–80 (2008).
Dellino, G. I. et al. Polycomb silencing blocks transcription initiation. Mol. Cell 13, 887–893 (2004).
Grau, D. J. et al. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 25, 2210–2221 (2011).
Eskeland, R. et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell 38, 452–464 (2010).
Gutiérrez, L. et al. The role of the histone H2A ubiquitinase Sce in Polycomb repression. Development 139, 117–127 (2012).
Schwartz, Y. B. et al. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nature Genet. 38, 700–705 (2006).
Lagarou, A. et al. dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing. Genes Dev. 22, 2799–2810 (2008). This study gives the first evidence of alternative protein complexes that contain core PRC1 components.
Farcas, A. M. et al. KDM2B links the Polycomb repressive complex 1 (PRC1) to recognition of CpG islands. eLife Sciences 1, e00205 (2012).
Wu, X., Johansen, J. V. & Helin, K. Fbxl10/Kdm2b recruits Polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell. 49, 1134–1146 (2013).
He, J. et al. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nature Cell Biol. 15, 373–384 (2013). References 12–14 shows that the zinc-finger-CxxC DNA-binding domain of KDM2B recruits a variant RING2 or RING1 complex to unmethylated CpG, thereby contributing to PcG-mediated repression in mouse embryonic stem cells.
Gao, Z. et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 45, 344–356 (2012). This key paper provides a comprehensive description of variant RING1 and RING2 protein complexes.
Tavares, L. et al. RYBP–PRC1 complexes mediate H2A ubiquitylation at Polycomb target sites independently of PRC2 and H3K27me3. Cell 148, 664–678 (2012).
Pfau, R. et al. Members of a family of JmjC domain-containing oncoproteins immortalize embryonic fibroblasts via a JmjC domain-dependent process. Proc. Natl Acad. Sci. USA 105, 1907–1912 (2008).
Tzatsos, A., Pfau, R., Kampranis, S. C. & Tsichlis, P. N. Ndy1/KDM2B immortalizes mouse embryonic fibroblasts by repressing the Ink4a/Arf locus. Proc. Natl Acad. Sci. USA 106, 2641–2646 (2009).
Wang, R. et al. Polycomb group targeting through different binding partners of RING1B C-terminal domain. Structure 18, 966–975 (2010).
Vandamme, J., Völkel, P., Rosnoblet, C., Le Faou, P. & Angrand, P.-O. Interaction proteomics analysis of Polycomb proteins defines distinct PRC1 complexes in mammalian cells. Mol. Cell. Proteomics 10, M110.002642 (2011).
Cao, R., Tsukada, Y.-I. & Zhang, Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 (2005).
Wei, J., Zhai, L., Xu, J. & Wang, H. Role of Bmi1 in H2A ubiquitylation and hox gene silencing. J. Biol. Chem. 281, 22537–22544 (2006).
Wu, X. et al. Cooperation between EZH2, NSPc1-mediated histone H2A ubiquitination and Dnmt1 in HOX gene silencing. Nucleic Acids Res. 36, 3590–3599 (2008).
Morey, L. et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell. 10, 47–62 (2012).
O'Loghlen, A. et al. MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation. Cell Stem Cell. 201210, 33–46 (2012).
Klauke, K. et al. Polycomb Cbx family members mediate the balance between haematopoietic stem cell self-renewal and differentiation. Nature Cell Biol. 15, 353–362 (2013).
Iwama, A. et al. Enhanced self-renewal of hematopoietic stem cells mediated by the Polycomb gene product Bmi-1. Immunity. 21, 843–851 (2004).
van der Lugt, N. M. et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 (1994).
Trojer, P. et al. L3MBTL2 protein acts in concert with PcG protein-mediated monoubiquitination of H2A to establish a repressive chromatin structure. Mol. Cell 42, 438–450 (2011).
Ogawa, H., Ishiguro, K., Gaubatz, S., Livingston, D. M. & Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 (2008).
O'Carroll, D. et al. The Polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell. Biol. 21, 4330–4336 (2001).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 (2011).
Kuzmichev, A., Jenuwein, T., Tempst, P. & Reinberg, D. Different Ezh2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol. Cell 14, 183–193 (2004).
Peters, A. H. F. M. et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 12, 1577–1589 (2003).
Jung, H. R., Pasini, D., Helin, K. & Jensen, O. N. Quantitative mass spectrometry of histones H3.2 and H3.3 in Suz12-deficient mouse embryonic stem cells reveals distinct, dynamic post-translational modifications at lys-27 and lys-36. Mol. Cell. Proteomics 9, 838–850 (2010).
Voigt, P. et al. Asymmetrically modified nucleosomes. Cell 151, 181–193 (2012).
Tie, F., Prasad-Sinha, J., Birve, A., Rasmuson-Lestander, Å. & Harte, P. J. A 1-megadalton ESC/E(Z) complex from Drosophila that contains Polycomblike and RPD3. Mol. Cell. Biol. 23, 3352–3362 (2003).
Nekrasov, M. et al. Pcl–PRC2 is needed to generate high levels of H3-K27 trimethylation at Polycomb target genes. EMBO J. 26, 4078–4088 (2007).
Sarma, K., Margueron, R., Ivanov, A., Pirrotta, V. & Reinberg, D. Ezh2 requires PHF1 to efficiently catalyze H3 lysine 27 trimethylation in vivo. Mol. Cell. Biol. 28, 2718–2731 (2008).
Cao, R. et al. Role of hPHF1 in H3K27 methylation and Hox gene silencing. Mol. Cell. Biol. 28, 1862–1872 (2008).
Ballaré, C. et al. Phf19 links methylated Lys36 of histone H3 to regulation of Polycomb activity. Nature Struct. Mol. Biol. 19, 1257–1265 (2012).
Cai, L. et al. An H3K36 methylation-engaging Tudor motif of Polycomb-like proteins mediates PRC2 complex targeting. Mol. Cell 49, 571–582 (2013).
Savla, U., Benes, J., Zhang, J. & Jones, R. S. Recruitment of Drosophila Polycomb-group proteins by Polycomblike, a component of a novel protein complex in larvae. Development 135, 813–817 (2008). References 39–44 combine the current knowledge of the features and roles of PRC2–PCL complexes.
Duncan, I. M. Polycomblike: a gene that appears to be required for the normal expression of the Bithorax and Antennapedia gene complexes of Drosophila melanogaster. Genetics 102, 49–70 (1982).
Papp, B. & Muller, J. Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins. Genes Dev. 20, 2041–2054 (2006).
Walker, E. et al. Polycomb-like 2 associates with PRC2 and regulates rranscriptional networks during mouse embryonic stem cell self-renewal and differentiation. Cell Stem Cell 6, 153–166 (2010).
Hunkapiller, J. et al. Polycomb-Like 3 promotes Polycomb repressive complex 2 binding to CpG Islands and embryonic stem cell self-renewal. PLoS Genet. 8, e1002576 (2012).
Musselman, C. A. et al. Molecular basis for H3K36me3 recognition by the Tudor domain of PHF1. Nature Struct. Mol. Biol. 19, 1266–1272 (2012).
Friberg, A., Oddone, A., Klymenko, T., Müller, J. & Sattler, M. Structure of an atypical Tudor domain in the Drosophila Polycomblike protein. Protein Sci. 19, 1906–1916 (2010).
Shen, X. et al. Jumonji modulates Polycomb activity and self-renewal versus differentiation of stem cells. Cell 139, 1303–1314 (2009).
Peng, J. C. et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139, 1290–1302 (2009).
Pasini, D. et al. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464, 306–310 (2010).
Li, G. et al. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 24, 368–380 (2010).
Landeira, D. et al. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nature Cell Biol. 12, 618–624 (2010).
Herz, H.-M. et al. Polycomb repressive complex 2-dependent and -independent functions of Jarid2 in transcriptional regulation in Drosophila. Mol. Cell. Biol. 32, 1683–1693 (2012). References 51–56 outline the properties of PRC2–JARID2 complexes.
Takeuchi, T. et al. Gene trap capture of a novel mouse gene, Jumonji, required for neural tube formation. Genes Dev. 9, 1211–1222 (1995).
Lee, Y. et al. Jumonji, a nuclear protein that is necessary for normal heart development. Circ. Res. 86, 932–938 (2000).
He, A. et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 26, 37–42 (2012).
Su, I.-h. et al. Polycomb group protein Ezh2 controls actin polymerization and cell signaling. Cell 121, 425–436 (2005).
Furuyama, T., Banerjee, R., Breen, T. R. & Harte, P. J. SIR2 is required for Polycomb silencing and is associated with an E(z) histone methyltransferase complex. Curr. Biol. 14, 1812–1821 (2004).
Kuzmichev, A. et al. Composition and histone substrates of Polycomb repressive group complexes change during cellular differentiation. Proc. Natl Acad. Sci. USA 102, 1859–1864 (2005).
Chan, C.-S., Rastelli, L. & Pirrotta, V. A. Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13, 2553–2564 (1994).
Müller, J. & Kassis, J. A. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr. Opin. Genet. Dev. 16, 476–484 (2006).
Horard, B., Tatout, C., Poux, S. & Pirrotta, V. Structure of a Polycomb response element and in vitro binding of Polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20, 3187–3197 (2000).
Kahn, T. G., Schwartz, Y. B., Dellino, G. I. & Pirrotta, V. Polycomb complexes and the propagation of the methylation mark at the Drosophila Ubx gene. J. Biol. Chem. 281, 29064–29075 (2006).
Kharchenko, P. V. et al. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471, 480–485 (2011).
Hodgson, J. W., Argiropoulos, B. & Brock, H. W. Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid Polycomb group response element-dependent silencing. Mol. Cell. Biol. 21, 4528–4543 (2001).
Dejardin, J. et al. Recruitment of Drosophila Polycomb group proteins to chromatin by DSP1. Nature 434, 533–538 (2005).
Ren, X., Vincenz, C. & Kerppola, T. K. Changes in the distributions and dynamics of Polycomb repressive complexes during embryonic stem cell differentiation. Mol. Cell. Biol. 28, 2884–2895 (2008).
Sing, A. et al. A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell 138, 885–897 (2009). This paper is the first report of the mammalian PRE-like element. In contrast to D. melanogaster PREs, this element can recruit PRC1 but not PRC2.
Woo, C. J., Kharchenko, P. V., Daheron, L., Park, P. J. & Kingston, R. E. A region of the human HOXD cluster that confers Polycomb-group responsiveness. Cell 140, 99–110 (2010).
Ku, M. et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genetics 4, e1000242 (2008).
Mendenhall, E. M. et al. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 6, e1001244 (2010).
Lynch, M. D. et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate Polycomb complex recruitment. EMBO J. 31, 317–329 (2012).
Thomson, J. P. et al. CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464, 1082–1086 (2010).
Blackledge, N. P. et al. CpG islands recruit a histone H3 lysine 36 demethylase. Mol. Cell 38, 179–190 (2010).
Dietrich, N. et al. REST-mediated recruitment of Polycomb repressor complexes in mammalian cells. PLoS Genet. 8, e1002494 (2012).
Arnold, P. et al. Modeling of epigenome dynamics identifies transcription factors that mediate Polycomb targeting. Genome Res. 23, 60–73 (2013).
Rinn, J. L. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007).
Tsai, M.-C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Gupta, R. A. et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 (2010).
Schorderet, P. & Duboule, D. Structural and functional differences in the long non-coding RNA Hotair in mouse and human. PLoS Genet. 7, e1002071 (2011).
Zhao, J., Sun, B. K., Erwin, J. A., Song, J.-J. & Lee, J. T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756 (2008).
Yap, K. L. et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by Polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell 38, 662–667 (2010).
Lee, J. T. Epigenetic regulation by long noncoding RNAs. Science 338, 1435–1439 (2012).
Khalil, A. M. et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl Acad. Sci. USA 106, 11667–11672 (2009).
Zhao, J. et al. Genome-wide identification of Polycomb-associated RNAs by RIP–seq. Mol. Cell 40, 939–953 (2010).
Kanhere, A. et al. Short RNAs are transcribed from repressed Polycomb target genes and Interact with Polycomb repressive complex-2. Mol. Cell 38, 675–688 (2010).
Ebert, A. et al. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18, 2973–2983 (2004).
Ketel, C. S. et al. Subunit contributions to histone methyltransferase activities of fly and worm Polycomb group complexes. Mol. Cell. Biol. 25, 6857–6868 (2005).
Schmitges, F. W. et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol. Cell 42, 330–341 (2011).
Yuan, W. et al. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J. Biol. Chem. 286, 7983–7989 (2011).
Yuan, W. et al. Dense chromatin activates Polycomb repressive complex 2 to regulate H3 lysine 27 methylation. Science 337, 971–975 (2012).
Margueron, R. et al. Role of the Polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767 (2009). References 92–95 report the effects of pre-existing H3K27, H3K36 and H3K4 methylation marks and of nucleosome density on the catalytic activity of PRC2.
Kagey, M. H., Melhuish, T. A. & Wotton, D. The Polycomb protein Pc2 is a SUMO E3. Cell 113, 127–137 (2003).
Kagey, M. H., Melhuish, T. A., Powers, S. E. & Wotton, D. Multiple activities contribute to Pc2 E3 function. EMBO J. 24, 108–119 (2005).
Zhang, H. et al. SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nature Genet. 36, 507–511 (2004).
Kang, X. et al. SUMO-specific protease 2 is essential for suppression of Polycomb group protein-mediated gene silencing during embryonic development. Mol. Cell 38, 191–201 (2010).
Ismail, I. H. et al. CBX4-mediated SUMO modification regulates BMI1 recruitment at sites of DNA damage. Nucleic Acids Res. 40, 5497–5510 (2012).
Voncken, J. W. et al. MAPKAP Kinase 3pK phosphorylates and regulates chromatin association of the Polycomb group protein Bmi1. J. Biol. Chem. 280, 5178–5187 (2005).
Elderkin, S. et al. A phosphorylated form of Mel-18 targets the Ring1B histone H2A ubiquitin ligase to chromatin. Mol. Cell 28, 107–120 (2007).
Schumacher, A., Faust, C. & Magnuson, T. Positional cloning of a global regulator of anterior-posterior patterning in mice. Nature 383, 250–253 (1996).
Voncken, J. W. et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc. Natl Acad. Sci. USA 100, 2468–2473 (2003).
van Lohuizen, M. et al. Identification of cooperating oncogenes in Eμ-myc transgenic mice by provirus tagging. Cell. 65, 737–752 (1991).
Sparmann, A. & van Lohuizen, M. Polycomb silencers control cell fate, development and cancer. Nature Rev. Cancer 6, 846–856 (2006).
Radulovic, V., de Haan, G. & Klauke, K. Polycomb-group proteins in hematopoietic stem cell regulation and hematopoietic neoplasms. Leukemia 27, 523–533 (2013).
Leung, C. et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428, 337–341 (2004).
Vonlanthen, S. et al. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A–ARF locus expression. Br. J. Cancer 84, 1372–1376 (2001).
Bruggeman, S. W. et al. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell 12, 328–341 (2007).
Gargiulo, G. et al. In vivo RNAi screen for bmi1 targets identifies TGF-β/BMP–ER stress pathways as key regulators of neural- and malignant glioma-stem cell homeostasis. Cancer Cell 23, 660–676 (2013).
Simon, J. A. & Lange, C. A. Roles of the Ezh2 histone methyltransferase in cancer epigenetics. Mut. Res. 647, 21–29 (2008).
Sneeringer, C. J. et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc. Natl Acad. Sci. USA 2010 107, 20980–20985 (2010).
McCabe, M. T. et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc. Natl Acad. Sci. USA 109, 2989–2994 (2012).
McCabe, M. T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Knutson, S. K. et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nature Chem. Biol. 8, 890–896 (2012). References 115 and 116 show that treatment with small-molecule inhibitors is a promising therapeutic strategy for lymphomas that have catalytically hyperactive variants of EZH2.
Simon, C. et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 26, 651–656 (2012).
Chan, K. M. et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 27, 985–990 (2013).
Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 (2013).
Xu, K. et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 338, 1465–1469 (2012). This paper reports a surprising PRC2-independent role of EZH2 in the proliferation of castration-resistant prostate cancer cells.
Lee, J. M. et al. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3-ubiquitin ligase complex. Mol. Cell 48, 572–586 (2012).
Shao, Z. et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 (1999).
Francis, N. J., Saurin, A. J., Shao, Z. & Kingston, R. E. Reconstitution of a functional core Polycomb repressive complex. Mol. Cell 8, 545–556 (2001).
Saurin, A. J., Shao, Z., Erdjument-Bromage, H., Tempst, P. & Kingston, R. E. A. Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412, 655–660 (2001).
Buchwald, G. et al. Structure and E3-ligase activity of the Ring–Ring complex of Polycomb proteins Bmi1 and Ring1b. EMBO J. 25, 2465–2474 (2006).
de Napoles, M. et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7, 663–676 (2004).
Wang, H. et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004).
Bornemann, D., Miller, E. & Simon, J. The Drosophila Polycomb group gene Sex comb on midleg (Scm) encodes a zinc finger protein with similarity to polyhomeotic protein. Development 122, 1621–1630 (1996).
Peterson, A. J. et al. A domain shared by the Polycomb group proteins Scm and Ph mediates heterotypic and homotypic interactions. Mol. Cell. Biol. 17, 6683–6692 (1997).
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Czermin, B. et al. Drosophila Enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185–196 (2002).
Müller, J. et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197–208 (2002).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of zeste protein. Genes Dev. 22, 2893–2905 (2002).
Pengelly, A. R., Copur, Ö., Jäckle, H., Herzig, A. & Müller, J. A histone mutant reproduces the phenotype caused by loss of histone-modifying factor Polycomb. Science 339, 698–699 (2013).
Cao, R. & Zhang, Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED–EZH2 complex. Mol. Cell 15, 57–67 (2004).
Kim, H., Kang, K. & Kim, J. AEBP2 as a potential targeting protein for Polycomb repression complex PRC2. Nucl. Acids Res. 37, 2940–2950 (2009).
Ciferri, C. et al. Molecular architecture of human Polycomb repre ssive complex 2. eLife 1, e00005 (2012).
Klymenko, T. et al. A Polycomb group protein complex with sequence-specific DNA-binding and selective methyl-lysine-binding activities. Genes Dev. 20, 1110–1122 (2006).
Oktaba, K. et al. Dynamic regulation by Polycomb group protein complexes controls pattern formation and the cell cycle in Drosophila. Develop. Cell 15, 877–889 (2008).
Mendenhall, E. M. et al. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 6, e1001244 (2010). This study shows that GC-rich elements that lack transcription activator-binding sites can autonomously recruit mammalian PRC2, even when these elements are derived from bacterial genomes.
Vella, P., Barozzi, I., Cuomo, A., Bonaldi, T. & Pasini, D. Yin Yang 1 extends the Myc-related transcription factors network in embryonic stem cells. Nucleic Acids Res. 40, 3403–3418 (2012).
Scheuermann, J. C. et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR–DUB. Nature 465, 243–247 (2010).
Yang, L. et al. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 147, 773–788 (2011).
Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. & Cavalli, G. Genome regulation by Polycomb and Trithorax proteins. Cell 128, 735–745 (2007).
Schwartz, Y. B. and Pirrotta, V. Polycomb silencing mechanisms and the management of genomic programmes. Nature Rev. Genet. 8, 9–22 (2007).
Acknowledgements
The work in the Y.B.S. laboratory is supported by grants from the Swedish Research Council, Carl Tryggers Foundation, Kempestiftelserna, Erik Philip-Sörensens Stiftelse and European Network of Excellence EpiGeneSys. The research of V.P. is supported by the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Homeotic genes
-
A set of related master transcription regulatory factors that regulate morphogenesis and tissue differentiation.
- CpG islands
-
Vertebrate genomic regions of the order of 1 kb that are rich in CpG dinucleotides; they often lack 5-methylcytosine and frequently correspond to promoter regions.
- Embryoid bodies
-
Three-dimensional aggregates of pluripotent stem cells.
- Paralogues
-
Genes that are originated by a duplication event within the genome.
- Orthologues
-
Genes in different species that are originated from a single gene of the last common ancestor.
Rights and permissions
About this article
Cite this article
Schwartz, Y., Pirrotta, V. A new world of Polycombs: unexpected partnerships and emerging functions. Nat Rev Genet 14, 853–864 (2013). https://doi.org/10.1038/nrg3603
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3603
This article is cited by
-
Phthalates impact on the epigenetic factors contributed specifically by the father at fertilization
Epigenetics & Chromatin (2023)
-
Plant BBR/BPC transcription factors: unlocking multilayered regulation in development, stress and immunity
Planta (2023)
-
CK2-mediated phosphorylation of SUZ12 promotes PRC2 function by stabilizing enzyme active site
Nature Communications (2022)
-
H2A ubiquitination is essential for Polycomb Repressive Complex 1-mediated gene regulation in Marchantia polymorpha
Genome Biology (2021)
-
Mapping of functional elements of the Fab-6 boundary involved in the regulation of the Abd-B hox gene in Drosophila melanogaster
Scientific Reports (2021)