Key Points
-
Single-molecule and genome-wide studies of transcription reveal the importance of dynamics for understanding the mechanisms of gene regulation.
-
A single gene can be regulated by dozens of factors interacting in a combinatorial manner. Observing all such interactions experimentally is still a daunting task, but computational models of transcription dynamics can provide insight into the underlying mechanisms.
-
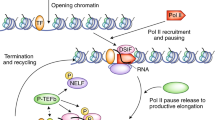
Molecular models of transcription often emphasize sequential, ordered recruitment, for example in the formation of a pre-initiation complex at a promoter. These sequential processes are best-described by non-equilibrium thermodynamics, in which kinetics and energy dependence are treated explicitly.
-
Models based on non-equilibrium thermodynamics provide insight into a range of transcription phenomena, including nucleosome positioning, transcriptional bursting, and refractory periods during transcription.
Abstract
Transcriptional regulation is achieved through combinatorial interactions between regulatory elements in the human genome and a vast range of factors that modulate the recruitment and activity of RNA polymerase. Experimental approaches for studying transcription in vivo now extend from single-molecule techniques to genome-wide measurements. Parallel to these developments is the need for testable quantitative and predictive models for understanding gene regulation. These conceptual models must also provide insight into the dynamics of transcription and the variability that is observed at the single-cell level. In this Review, we discuss recent results on transcriptional regulation and also the models those results engender. We show how a non-equilibrium description informs our view of transcription by explicitly considering time- and energy-dependence at the molecular level.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
McNally, J. G., Muller, W. G., Walker, D., Wolford, R. & Hager, G. L. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287, 1262–1265 (2000).
Dundr, M. et al. A kinetic framework for a mammalian RNA polymerase in vivo. Science 298, 1623–1626 (2002).
Dion, M. F. et al. Dynamics of replication-independent histone turnover in budding yeast. Science 315, 1405–1408 (2007). This study measured the turnover rates of core histones on a genome-wide scale and reported dwell times on the order of tens of minutes that varied substantially between genomic locations.
Métivier, R., Penot, G., Hübner, M. R., Reid, G. & Brand, H. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763 (2003).
Chubb, J. R., Trcek, T., Shenoy, S. M. & Singer, R. H. Transcriptional pulsing of a developmental gene. Curr. Biol. 16, 1018–1025 (2006).
Larson, D. R. What do expression dynamics tell us about the mechanism of transcription? Curr. Op. Genet. Dev. 21, 591–599 (2011).
Stamatoyannopoulos, J. A. What does our genome encode? Genome Res. 22, 1602–1611 (2012).
Johnson, D. S., Mortazavi, A., Myers, R. M. & Wold, B. Genome-wide mapping of in vivo protein-DNA interactions. Science 316, 1497–1502 (2007). The first article to report genome-wide binding of a chromatin-interacting factor by ChIP followed by high-throughput sequencing (ChIP–seq).
Rhee, H. S. & Pugh, B. F. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell 147, 1408–1419 (2011).
The ENCODE Project Consortium. ENCODE project [online].
van Royen, M. E., Zotter, A., Ibrahim, S. M., Geverts, B. & Houtsmuller, A. B. Nuclear proteins: finding and binding target sites in chromatin. Chromosome Res. 19, 83–98 (2011).
Raj, A. & van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226 (2008).
Soutoglou, E. & Talianidis, I. Coordination of PIC assembly and chromatin remodeling during differentiation-induced gene activation. Science 295, 1901–1904 (2002).
Shang, Y. F., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
Sharma, D. & Fondell, J. D. Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc. Natl Acad. Sci. USA 99, 7934–7939 (2002).
Métivier, R. et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 452, 45–50 (2008).
Elf, J., Li, G. W. & Xie, X. S. Probing transcription factor dynamics at the single-molecule level in a living cell. Science 316, 1191–1194 (2007).
Larson, D. R., Zenklusen, D., Wu, B., Chao, J. A. & Singer, R. H. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 332, 475–478 (2011). The first direct observation of RNA synthesis in living cells at the single-transcript level, which revealed the kinetics of initiation and elongation.
Fuda, N. J., Ardehali, M. B. & Lis, J. T. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192 (2009).
Cairns, B. R. The logic of chromatin architecture and remodelling at promoters. Nature 461, 193–198 (2009).
Weake, V. M. & Workman, J. L. Inducible gene expression: diverse regulatory mechanisms. Nature Rev. Genet. 11, 426–437 (2010).
Buratowski, S., Hahn, S., Guarente, L. & Sharp, P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56, 549–561 (1989).
Roeder, R. G. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579, 909–915 (2005).
Thomas, M. C. & Chiang, C.-M. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41, 105–178 (2006).
Dieci, G. & Sentenac, A. Detours and shortcuts to transcription reinitiation. Trends Biochem. Sci. 28, 202–209 (2003).
Esnault, C. et al. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol. Cell 31, 337–346 (2008).
Sikorski, T. W. & Buratowski, S. The basal initiation machinery: beyond the general transcription factors. Curr. Opin. Cell Biol. 21, 344–351 (2009).
Kim, S., Shevde, N. K. & Pike, J. W. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J. Bone Miner. Res. 20, 305–317 (2004).
Nagaich, A. K., Walker, D. A., Wolford, R. & Hager, G. L. Rapid periodic binding and displacement of the glucocorticoid receptor during chromatin remodeling. Mol. Cell 14, 163–174 (2004). This study presented a minimalist in vitro system that recapitulated the recruitment of a remodeller to a chromatin template by GR, thus resulting in its own eviction following remodelling. This system displays an intrinsic periodic behaviour.
Qiu, Y. et al. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell 22, 669–679 (2006).
John, S. et al. Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology 150, 1766–1774 (2009).
Li, G. et al. Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo. Mol. Cell 38, 41–53 (2010).
Carroll, J. S. et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 (2005).
Laganière, J. et al. Location analysis of estrogen receptor α target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl Acad. Sci. USA 102, 11651–11656 (2005).
Lupien, M. et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132, 958–970 (2008).
Welboren, W.-J. et al. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 28, 1418–1428 (2009).
Hurtado, A., Holmes, K. A., Ross-Innes, C. S., Schmidt, D. & Carroll, J. S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nature Genet. 43, 27–33 (2010).
Kong, S. L., Li, G., Loh, S. L., Sung, W.-K. & Liu, E. T. Cellular reprogramming by the conjoint action of ERα, FOXA1, and GATA3 to a ligand-inducible growth state. Mol. Syst. Biol. 7, 526 (2011).
Cirillo, L. A. et al. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 17, 244–254 (1998).
Cirillo, L. A. et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002).
Perlmann, T., Eriksson, P. & Wrange, O. Quantitative analysis of the glucocorticoid receptor-DNA interaction at the mouse mammary tumor virus glucocorticoid response element. J. Biol. Chem. 265, 17222–17229 (1990).
Zabel, U. & Baeuerle, P. A. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell 61, 255–265 (1990).
Lieberman, B. A. & Nordeen, S. K. DNA intersegment transfer, how steroid receptors search for a target site. J. Biol. Chem. 272, 1061–1068 (1997).
Yie, J. M., Merika, M., Munshi, N., Chen, G. Y. & Thanos, D. The role of HMG I(Y) in the assembly and function of the IFN-β enhanceosome. EMBO J. 18, 3074–3089 (1999).
Stenoien, D. L. et al. FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nature Cell Biol. 3, 15–23 (2001).
Lickwar, C. R., Mueller, F., Hanlon, S. E., McNally, J. G. & Lieb, J. D. Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 484, 251–255 (2012). An assessment of genome-wide turnover of a transcription factor revealing that gene output correlates better with dwell time at regulatory sites than with average occupancy.
Normanno, D., Dahan, M. & Darzacq, X. Intra-nuclear mobility and target search mechanisms of transcription factors: a single-molecule perspective on gene expression. Biochim. Biophys. Acta Gene Regulatory Mechs. 1819, 482–493 (2012).
Darzacq, X. et al. Imaging transcription in living cells. Annu. Rev. Biophys. 38, 173–196 (2009).
Sung, M.-H. & McNally, J. G. Live cell imaging and systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 167–182 (2010).
Hager, G. L., McNally, J. G. & Misteli, T. Transcription dynamics. Mol. Cell 35, 741–753 (2009).
Mueller, F., Mazza, D., Stasevich, T. J. & McNally, J. G. FRAP and kinetic modeling in the analysis of nuclear protein dynamics: what do we really know? Curr. Opin. Cell Biol. 22, 403–411 (2010).
Stasevich, T. J. et al. Cross-validating FRAP and FCS to quantify the impact of photobleaching on in vivo binding estimates. Biophys. J. 99, 3093–3101 (2010).
Nalley, K., Johnston, S. A. & Kodadek, T. Proteolytic turnover of the Gal4 transcription factor is not required for function in vivo. Nature 442, 1054–1057 (2006).
Deal, R. B., Henikoff, J. G. & Henikoff, S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science 328, 1161–1164 (2010).
Rayasam, G. V. et al. Ligand-specific dynamics of the progesterone receptor in living cells and during chromatin remodeling in vitro. Mol. Cell. Biol. 25, 2406–2418 (2005).
Agresti, A., Scaffidi, P., Riva, A., Caiolfa, V. R. & Bianchi, M. E. GR and HMGB1 interact only within chromatin and influence each other's residence time. Mol. Cell 18, 109–121 (2005).
Yao, J., Munson, K. M., Webb, W. W. & Lis, J. T. Dynamics of heat shock factor association with native gene loci in living cells. Nature 442, 1050–1053 (2006).
Darzacq, X. et al. In vivo dynamics of RNA polymerase II transcription. Nature Struct. Mol. Biol. 14, 796–806 (2007).
Gorski, S. A., Snyder, S. K., John, S., Grummt, I. & Misteli, T. Modulation of RNA polymerase assembly dynamics in transcriptional regulation. Mol. Cell 30, 486–497 (2008).
Sprouse, R. O. et al. Regulation of TATA-binding protein dynamics in living yeast cells. Proc. Natl Acad. Sci. USA 105, 13304–13308 (2008).
Erdel, F., Schubert, T., Marth, C., Längst, G. & Rippe, K. Human ISWI chromatin-remodeling complexes sample nucleosomes via transient binding reactions and become immobilized at active sites. Proc. Natl Acad. Sci. USA 107, 19873–19878 (2010).
Harper, C. V. et al. Dynamic analysis of stochastic transcription cycles. PLoS Biol. 9, e1000607 (2011). This paper presents inference of single-gene activity from fluctuations of protein expression levels. Two identical genes monitored simultaneously in the same cell display uncorrelated periodic activity, thus indicating a cis -originating mechanism that is consistent with a molecular ratchet.
Suter, D. M. et al. Mammalian genes are transcribed with widely different bursting kinetics. Science 332, 472–474 (2011). This study presents inference of single-gene activity from fluctuations of protein expression levels. Distributions of active and inactive times in gene bursting indicate refractoriness in gene reactivation, thus reflecting a multistep sequential process.
Levsky, J. M. & Singer, R. H. Gene expression and the myth of the average cell. Trends Cell Biol. 13, 4–6 (2003).
Raj, A., Peskin, C. S., Tranchina, D., Vargas, D. Y. & Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 4, e309 (2006).
Karpova, T. S. et al. Concurrent fast and slow cycling of a transcriptional activator at an endogenous promoter. Science 319, 466–469 (2008).
Voss, T. C. et al. Combinatorial probabilistic chromatin interactions produce transcriptional heterogeneity. J. Cell Sci. 122, 345–356 (2009).
Viñuelas, J. et al. Quantifying the contribution of chromatin dynamics to stochastic gene expression reveals long, locus-dependent periods between transcriptional bursts. BMC Biol. 11, 15 (2013).
von Hippel, P. H. From “simple” DNA-protein interactions to the macromolecular machines of gene expression. Annu. Rev. Biophys. Biomol. Struct. 36, 79–105 (2007).
Field, Y. et al. Distinct modes of regulation by chromatin encoded through nucleosome positioning signals. PLoS Comput. Biol. 4, e1000216 (2008).
Raveh-Sadka, T., Levo, M. & Segal, E. Incorporating nucleosomes into thermodynamic models of transcription regulation. Genome Res. 19, 1480–1496 (2009).
Segal, E. & Widom, J. From DNA sequence to transcriptional behaviour: a quantitative approach. Nature Rev. Genet. 10, 443–456 (2009).
Lam, F. H., Steger, D. J. & O'Shea, E. K. Chromatin decouples promoter threshold from dynamic range. Nature 453, 246–250 (2008).
Kingston, R. E. & Narlikar, G. J. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes. Dev. 13, 2339–2352 (1999).
Partensky, P. D. & Narlikar, G. J. Chromatin remodelers act globally, sequence positions nucleosomes locally. J. Mol. Biol. 391, 12–25 (2009).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Clapier, C. R. & Cairns, B. R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 (2009).
Lemaire, V., Lee, C., Lei, J., Métivier, R. & Glass, L. . Sequential recruitment and combinatorial assembling of multiprotein complexes in transcriptional activation. Phys. Rev. Lett. 96, 198102 (2006).
Coulon, A., Gandrillon, O. & Beslon, G. On the spontaneous stochastic dynamics of a single gene: complexity of the molecular interplay at the promoter. BMC Systems Biol. 4, 2 (2010).
Kim, H. D. & O'Shea, E. K. A quantitative model of transcription factor-activated gene expression. Nature Struct. Mol. Biol. 15, 1192–1198 (2008).
Boettiger, A. N., Ralph, P. L. & Evans, S. N. Transcriptional regulation: effects of promoter proximal pausing on speed, synchrony and reliability. PLoS Comput. Biol. 7, e1001136 (2011).
Schwabe, A., Rybakova, K. N. & Bruggeman, F. J. Transcription stochasticity of complex gene regulation models. Biophys. J. 103, 1152–1161 (2012).
Dobrzynski, M. & Bruggeman, F. J. Elongation dynamics shape bursty transcription and translation. Proc. Natl Acad. Sci. USA 106, 2583–2588 (2009).
Sanchez, A. & Kondev, J. Transcriptional control of noise in gene expression. Proc. Natl Acad. Sci. USA 105, 5081–5086 (2008).
Boeger, H., Griesenbeck, J. & Kornberg, R. D. Nucleosome retention and the stochastic nature of promoter chromatin remodeling for transcription. Cell 133, 716–726 (2008).
Müller, D. & Stelling, J. Precise regulation of gene expression dynamics favors complex promoter architectures. PLoS Comput. Biol. 5, e1000279 (2009).
Kaplan, N. et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458, 362–366 (2009).
Valouev, A. et al. Determinants of nucleosome organization in primary human cells. Nature 474, 516–520 (2011).
Yuan, G. C. et al. Genome-scale identification of nucleosome positions in S. cerevisiae. Science 309, 626–630 (2005).
Jiang, C. & Pugh, B. F. Nucleosome positioning and gene regulation: advances through genomics. Nature Rev. Genet. 10, 161–172 (2009).
Segal, E. et al. A genomic code for nucleosome positioning. Nature 442, 772–778 (2006).
Kornberg, R. D. & Stryer, L. Statistical distributions of nucleosomes: nonrandom locations by a stochastic mechanism. Nucleic Acids Res. 16, 6677–6690 (1988).
Vaillant, C., Audit, B. & Arneodo, A. Experiments confirm the influence of genome long-range correlations on nucleosome positioning. Phys. Rev. Lett. 99, 218103 (2007).
Chevereau, G., Palmeira, L., Thermes, C., Arneodo, A. & Vaillant, C. Thermodynamics of intragenic nucleosome ordering. Phys. Rev. Lett. 103, 188103 (2009).
Weiner, A., Hughes, A., Yassour, M., Rando, O. J. & Friedman, N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 20, 90–100 (2010).
Zhang, Z. et al. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science 332, 977–980 (2011). This study reports an in vitro reconstitution of nucleosome positioning patterns over gene bodies that mimic in vivo positions. The need for ATP to obtain this result demonstrates the non-equilibrium nature of nucleosome positioning.
Sprague, B. L. et al. Analysis of binding at a single spatially localized cluster of binding sites by fluorescence recovery after photobleaching. Biophys. J. 91, 1169–1191 (2006).
Linder, B. Thermodynamics and introductory statistical mechanics (LibreDigital, 2004).
Elbi, C. et al. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc. Natl Acad. Sci. USA 101, 2876–2881 (2004).
Stavreva, D. A., Muller, W. G., Hager, G. L., Smith, C. L. & McNally, J. G. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol. Cell. Biol. 24, 2682–2697 (2004).
Bosisio, D. et al. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-κB-dependent gene activity. EMBO J. 25, 798–810 (2006).
Karpova, T. S., Chen, T. Y., Sprague, B. L. & McNally, J. G. Dynamic interactions of a transcription factor with DNA are accelerated by a chromatin remodeller. EMBO Rep. 5, 1064–1070 (2004).
Johnson, T. A., Elbi, C., Parekh, B. S., Hager, G. L. & John, S. Chromatin remodeling complexes interact dynamically with a glucocorticoid receptor-regulated promoter. Mol. Biol. Cell 19, 3308–3322 (2008).
Fletcher, T. M. et al. ATP-dependent mobilization of the glucocorticoid receptor during chromatin remodeling. Mol. Cell. Biol. 22, 3255–3263 (2002).
Voss, T. C. et al. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell 146, 544–554 (2011). A demonstration that GR never saturates its response elements and that its transient binding modifies the chromatin, thus promoting the subsequent associations of other factors.
Ko, M. S., Nakauchi, H. & Takahashi, N. The dose dependence of glucocorticoid-inducible gene expression results from changes in the number of transcriptionally active templates. EMBO J. 9, 2835–2842 (1990).
Walters, M. C. et al. Enhancers increase the probability but not the level of gene expression. Proc. Natl Acad. Sci. USA 92, 7125–7129 (1995).
White, M. R. et al. Real-time analysis of the transcriptional regulation of HIV and hCMV promoters in single mammalian cells. J. Cell Sci. 108, 441–455 (1995).
Paszek, P. et al. Population robustness arising from cellular heterogeneity. Proc. Natl Acad. Sci. USA 107, 11644–11649 (2010).
Raj, A., Rifkin, S. A., Andersen, E. & van Oudenaarden, A. Variability in gene expression underlies incomplete penetrance. Nature 463, 913–918 (2010).
Swain, P. S., Elowitz, M. B. & Siggia, E. D. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc. Natl Acad. Sci. USA 99, 12795–12800 (2002).
Raser, J. M. Control of stochasticity in eukaryotic gene expression. Science 304, 1811–1814 (2004).
Paulsson, J. Summing up the noise in gene networks. Nature 427, 415–418 (2004).
Paulsson, J. Models of stochastic gene expression. Phys. Life Rev. 2, 157–175 (2005).
Becskei, A., Kaufmann, B. B. & van Oudenaarden, A. Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nature Genet. 37, 937–944 (2005).
Sigal, A. et al. Variability and memory of protein levels in human cells. Nature 444, 643–646 (2006).
Skupsky, R., Burnett, J. C., Foley, J. E., Schaffer, D. V. & Arkin, A. P. HIV promoter integration site primarily modulates transcriptional burst size rather than frequency. PLoS Comput. Biol. 6, e1000952 (2010).
Stevense, M., Muramoto, T., Muller, I. & Chubb, J. R. Digital nature of the immediate-early transcriptional response. Development 137, 579–584 (2010).
Muramoto, T., Müller, I., Thomas, G., Melvin, A. & Chubb, J. R. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr. Biol. 20, 397–406 (2010).
Peccoud, J. & Ycart, B. Markovian modeling of gene-product synthesis. Theor. Popul. Biol. 48, 222–234 (1995).
Lionnet, T., Wu, B., Grünwald, D., Singer, R. H. & Larson, D. R. Nuclear physics: quantitative single-cell approaches to nuclear organization and gene expression. Cold Spring Harb. Symp. Quant. Biol. 75, 113–126 (2010).
Zenklusen, D., Larson, D. R. & Singer, R. H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nature Struct. Mol. Biol. 15, 1263–1271 (2008).
Golding, I. Decision making in living cells: lessons from a simple system. Annu. Rev. Biophys. 40, 63–80 (2011).
Newman, J. R. S. et al. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441, 840–846 (2006).
Gandhi, S. J., Zenklusen, D., Lionnet, T. & Singer, R. H. Transcription of functionally related constitutive genes is not coordinated. Nature Struct. Mol. Biol. 18, 27–34 (2010).
So, L.-h. et al. General properties of transcriptional time series in Escherichia coli. Nature Genet. 43, 554–560 (2011).
Dunlop, M. J., Cox, R. S., Levine, J. H., Murray, R. M. & Elowitz, M. B. Regulatory activity revealed by dynamic correlations in gene expression noise. Nature Genet. 40, 1493–1498 (2008).
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998). The first observation of RNA particles in living cells using the MS2 RNA-labelling technique.
Larson, D. R., Singer, R. H. & Zenklusen, D. A single molecule view of gene expression. Trends Cell Biol. 19, 630–637 (2009).
Grünwald, D. & Singer, R. H. In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport. Nature 467, 604–607 (2010).
Yunger, S., Rosenfeld, L., Garini, Y. & Shav-Tal, Y. Single-allele analysis of transcription kinetics in living mammalian cells. Nature Methods 7, 631–633 (2010).
Lionnet, T. et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nature Methods 8, 165–170 (2011).
Schmidt, U. et al. Real-time imaging of cotranscriptional splicing reveals a kinetic model that reduces noise: implications for alternative splicing regulation. J. Cell Biol. 193, 819–829 (2011).
Bar-Even, A. et al. Noise in protein expression scales with natural protein abundance. Nature Genet. 38, 636–643 (2006).
Blake, W. J., Kærn, M., Cantor, C. R. & Collins, J. J. Noise in eukaryotic gene expression. Nature 422, 633–637 (2003).
Batenchuk, C. et al. Chromosomal position effects are linked to Sir2-mediated variation in transcriptional burst size. Biophys. J. 100, L56–L58 (2011).
Singh, A., Razooky, B., Cox, C. D., Simpson, M. L. & Weinberger, L. S. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys. J. 98, L32–L34 (2010).
Pedraza, J. M. & Paulsson, J. Effects of molecular memory and bursting on fluctuations in gene expression. Science 319, 339–343 (2008).
Métivier, R., Reid, G. & Gannon, F. Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Rep. 7, 161–167 (2006).
Moffitt, J. R. et al. Intersubunit coordination in a homomeric ring ATPase. Nature 457, 446–451 (2009).
Arazi, A., Ben-Jacob, E. & Yechiali, U. Bridging genetic networks and queueing theory. Phys. A: Statist. Mechan. Appl. 332, 585–616 (2004).
Weber, A., Liu, J. H., Collins, I. & Levens, D. TFIIH operates through an expanded proximal promoter to fine-tune c-myc expression. Mol. Cell. Biol. 25, 147–161 (2005).
Kimura, H., Sugaya, K. & Cook, P. R. The transcription cycle of RNA polymerase II in living cells. J. Cell Biol. 159, 777–782 (2002).
Singh, J. & Padgett, R. A. Rates of in situ transcription and splicing in large human genes. Nature Struct. Mol. Biol. 16, 1128–1133 (2009).
Hager, G. L. et al. Chromatin dynamics and the evolution of alternate promoter states. Chromosome Res. 14, 107–116 (2006).
Reid, G., Gallais, R. & Métivier, R. Marking time: the dynamic role of chromatin and covalent modification in transcription. Int. J. Biochem. Cell Biol. 41, 155–163 (2009).
Baddeley, A. Working memory: looking back and looking forward. Nature Rev. Neurosci. 4, 829–839 (2003).
Ong, K. M., Blackford, J. A., Kagan, B. L., Simons, S. S. & Chow, C. C. A theoretical framework for gene induction and experimental comparisons. Proc. Natl Acad. Sci. USA 107, 7107–7112 (2010).
Kininis, M. et al. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol. Cell. Biol. 27, 5090–5104 (2007).
Kang, Z., Pirskanen, A., Jänne, O. A. & Palvimo, J. J. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J. Biol. Chem. 277, 48366–48371 (2002).
Paszek, P., Jackson, D. A. & White, M. R. Oscillatory control of signalling molecules. Curr. Opin. Genet. Dev. 20, 670–676 (2010).
Ferrell, J. E., Tsai, T. Y.-C. & Yang, Q. Modeling the cell cycle: why do certain circuits oscillate? Cell 144, 874–885 (2011).
Thurman, R. E. et al. The accessible chromatin landscape of the human genome. Nature 488, 75–82 (2012).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Simons, S. S. & Chow, C. C. The road less traveled: New views of steroid receptor action from the path of dose-response curves. Mol. Cell. Endocrinol. 348, 373–382 (2011).
Acknowledgements
We would like to thank the members of the Transcription Imaging Consortium at the Janelia Farm Research Campus for their important contributions to the theories proposed here. We also thank B. Lewis for critical reading of the manuscript. This work is supported in part by funding from the US National Institutes of Health (NIH) grants GM57071, 84364 and 86217 to R.H.S. D.R.L. is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Glossary
- Chromatin remodellers
-
Along with chromatin modifiers, these are complexes and enzymes that affect the status of chromatin, through conformational changes (such as nucleosome displacement or eviction, or histone replacement) or by depositing or removing covalent marks on histone tails. These processes are accompanied by the hydrolysis of coenzymes, releasing chemical energy.
- Chromatin immunoprecipitation
-
(ChIP). A method to assess the occupancy at a given genomic locus by a particular factor. It is carried out by amplifying DNA fragments that have been crosslinked to the factor of interest and pulled down using an antibody.
- Re-ChIP
-
A modification of the chromatin immunoprecipitation (ChIP) method. By successively using several antibodies, it allows an assessment of the co-occupancy of a locus by multiple factors.
- Equilibrium
-
For a chemical system to be at equilibrium, every reaction must occur in both directions with equal probability (or rate). Hence, a system can reach a steady state without ever being at equilibrium.
- Steady state
-
Refers to a system that does not evolve over time. This concept may apply to various descriptions such as a set of concentrations of molecular species or the probability distribution of a set of features among a population of cells (for example, nucleosome positions on a specific promoter).
- Fluorescence recovery after photobleaching
-
(FRAP). An experimental microscopy method to assess the mobility of molecules in living cells. In FRAP, the rate at which fluorescently labelled molecules repopulate a region of the cell that has been photobleached reflects both their diffusion and binding to chromatin.
- Fluorescence correlation spectroscopy
-
(FCS). An experimental microscopy method to assess the mobility of molecules in living cells. In FCS, those properties are derived from the temporal fluctuations of fluorescence due to molecules entering and leaving a small optically defined volume of the cell.
- MS2 and/or PP7 RNA labelling
-
A microscopy technique for labelling, in live cells, the transcripts from an artificial gene construct. Many molecules of fluorescent proteins (MS2 or PP7) bind each RNA on a specific cassette, thus allowing the monitoring of the number of nascent RNAs being transcribed at the gene locus over time.
- Genomic nuclear run-on followed by high-throughput sequencing
-
(GRO-seq). This approach uses nuclear run-on methodology to map transcriptionally engaged polymerases on a genome-wide level. This approach constitutes a direct measure of transcriptional activity.
- Flux
-
The net flux of a reaction is the difference between the rates at which it is observed to occur in one direction versus the other. When a system satisfies detailed balance, the net fluxes of all reactions are null.
- Equilibrium constant
-
The ratio of association and dissociation rates. In an equilibrium context, this describes the affinity of a molecule for a binding site and directly relates to interaction energy. In a non-equilibrium context, such a ratio does not reflect the interaction energy and should not be called an equilibrium constant
- Refractoriness
-
A time distribution function that displays an increasing phase for short delays is said to be refractory because — as opposed to an exponential distribution — the probability for the random event to occur is not constant but increases over time, thus shaping the distribution and reflecting the underlying biomolecular mechanics.
- Memoryless
-
A process is memoryless if the time it takes to complete is exponentially distributed, indicating that its probability of completion does not change over time and is hence independent of the past. A succession of memoryless events (for example, sequential recruitment of factors) can lead to memory.
- Single-molecule fluorescence in situ hybridization
-
(smFISH). A microscopy technique for labelling and visualizing RNA in fixed cells using many probes that are hybridized to a single transcript. For each cell, this technique allows the counting of the number of RNAs at the transcription site (that is, nascent RNAs) and also the number of RNAs in the cell.
- Hill coefficient
-
A value describing the steepness of a dose–response curve at the level of transition between a low value and a high response. It reflects the level of cooperativity in the binding of the regulator and equals 1 in the case of uncooperative binding.
- Metastable
-
A biochemical or conformational feature can be qualified as metastable if it is very unlikely to disappear spontaneously without the intervention of an energy- dependent enzymatic reaction. For example, post-transcriptional modifications of histone tails are metastable, as are certain conformational or remodelled states of chromatin.
Rights and permissions
About this article
Cite this article
Coulon, A., Chow, C., Singer, R. et al. Eukaryotic transcriptional dynamics: from single molecules to cell populations. Nat Rev Genet 14, 572–584 (2013). https://doi.org/10.1038/nrg3484
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3484
This article is cited by
-
Nonlinear control of transcription through enhancer–promoter interactions
Nature (2022)
-
Modulating gene regulation function by chemically controlled transcription factor clustering
Nature Communications (2022)
-
Structure of the p53/RNA polymerase II assembly
Communications Biology (2021)
-
Live-cell imaging reveals the spatiotemporal organization of endogenous RNA polymerase II phosphorylation at a single gene
Nature Communications (2021)
-
A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes
Scientific Reports (2021)