Key Points
-
DNA replication in mammalian nuclei is carried out in specialized structures that are known as replication factories. Throughout S phase, these factories assemble dynamically, and replicons that are located in their vicinity are recruited to the replication sub-compartment.
-
GC-rich, gene-dense regions tend to replicate early in S phase, whereas AT-rich regions that are usually gene-poor replicate late. In many cases, expressed genes replicate early in S phase and silent genes replicate late, but there are many exceptions to this rule.
-
Early replication timing is probably determined by epigenetic information and chromatin structure rather than by individual gene expression per se. Gene expression does not seem to be a direct consequence of early replication.
-
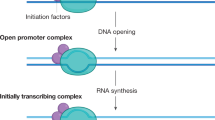
Transcription is highly compartmentalized in mammalian nuclei. Nascent transcription by RNA polymerase II occurs in foci that are known as transcription factories. Cells contain very few transcription factories compared with the number of active genes.
-
Most 'active' genes are not continuously transcribed, but undergo transcription cycles. Modulation of expression might occur by changing the frequency or duration of the 'on' state versus the 'off' state.
-
On activation, genes migrate to pre-assembled transcription factories that are shared with other actively transcribed genes.
-
In higher eukaryotes approximately 15% of the genome is transcribed. Most transcribed genomic regions are intergenic sequences.
-
The finding that most of the genome is transcribed indicates that transcription factories are principal focal points for the nuclear organization of the genome.
-
The clustering of genes around functional sites in the nucleus might impose selective pressures on the organization of genes and regulatory elements at the primary sequence level, thereby contributing to the observed clustering of highly expressed genes in the genome.
Abstract
As the relationship between nuclear structure and function begins to unfold, a picture is emerging of a dynamic landscape that is centred on the two main processes that execute the regulated use and propagation of the genome. Rather than being subservient enzymatic activities, the replication and transcriptional machineries provide potent forces that organize the genome in three-dimensional nuclear space. Their activities provide opportunities for epigenetic changes that are required for differentiation and development. In addition, they impose physical constraints on the genome that might help to shape its evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McNairn, A. J. & Gilbert, D. M. Epigenomic replication: linking epigenetics to DNA replication. Bioessays 25, 647–656 (2003).
Goren, A. & Cedar, H. Replicating by the clock. Nature Rev. Mol. Cell Biol. 4, 25–32 (2003).
Gilbert, D. M. Replication timing and transcriptional control: beyond cause and effect. Curr. Opin. Cell Biol. 14, 377–383 (2002).
Schubeler, D. et al. Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nature Genet. 32, 438–442 (2002).
Woodfine, K. et al. Replication timing of the human genome. Hum. Mol. Genet. 13, 191–202 (2004).
Cheng, J. et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 308, 1149–1154 (2005). An extensive and unbiased study showing that the human transcriptome is composed of a complex network of overlapping poly(A)+, poly(A)− and bimorphic RNA species, most of which are non-coding.
Kapranov, P. et al. Large-scale transcriptional activity in chromosomes 21 and 22. Science 296, 916–919 (2002).
Peterson, C. L. & Laniel, M. A. Histones and histone modifications. Curr. Biol. 14, R546–R551 (2004).
Khorasanizadeh, S. The nucleosome: from genomic organization to genomic regulation. Cell 116, 259–272 (2004).
Elgin, S. C. & Grewal, S. I. Heterochromatin: silence is golden. Curr. Biol. 13, R895–R898 (2003).
Hatton, K. S. et al. Replication program of active and inactive multigene families in mammalian cells. Mol. Cell. Biol. 8, 2149–2158 (1988).
Diffley, J. F. Regulation of early events in chromosome replication. Curr. Biol. 14, R778–R786 (2004).
Ma, H. et al. Spatial and temporal dynamics of DNA replication sites in mammalian cells. J. Cell Biol. 143, 1415–1425 (1998).
Jackson, D. A. & Pombo, A. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J. Cell Biol. 140, 1285–1295 (1998).
Philimonenko, A. A. et al. Dynamics of DNA replication: an ultrastructural study. J. Struct. Biol. 148, 279–289 (2004).
Quivy, J. P. et al. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 23, 3516–3526 (2004).
Sadoni, N., Cardoso, M. C., Stelzer, E. H., Leonhardt, H. & Zink, D. Stable chromosomal units determine the spatial and temporal organization of DNA replication. J. Cell Sci. 117, 5353–5365 (2004). The authors describe elegant experiments in live cells showing that during S-phase progression, replication sequentially proceeds through spatially adjacent sets of chromosomal domains.
Sporbert, A., Gahl, A., Ankerhold, R., Leonhardt, H. & Cardoso, M. C. DNA polymerase clamp shows little turnover at established replication sites but sequential de novo assembly at adjacent origin clusters. Mol. Cell 10, 1355–1365 (2002).
Abney, J. R., Cutler, B., Fillbach, M. L., Axelrod, D. & Scalettar, B. A. Chromatin dynamics in interphase nuclei and its implications for nuclear structure. J. Cell Biol. 137, 1459–1468 (1997).
Chubb, J. R., Boyle, S., Perry, P. & Bickmore, W. A. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr. Biol. 12, 439–445 (2002). This report shows that nuclear substructures, including the nucleolus and nuclear periphery, constrain the movements of chromatin and have a role in the three-dimensional organization of chromatin in the nucleus.
Molenaar, C. et al. Visualizing telomere dynamics in living mammalian cells using PNA probes. EMBO J. 22, 6631–6641 (2003).
MacAlpine, D. M., Rodriguez, H. K. & Bell, S. P. Coordination of replication and transcription along a Drosophila chromosome. Genes Dev. 18, 3094–3105 (2004).
Azuara, V. et al. Heritable gene silencing in lymphocytes delays chromatid resolution without affecting the timing of DNA replication. Nature Cell Biol. 5, 668–674 (2003).
Belyakin, S. N. et al. Genomic analysis of Drosophila chromosome underreplication reveals a link between replication control and transcriptional territories. Proc. Natl Acad. Sci. USA 102, 8269–8274 (2005).
Hiratani, I., Leskovar, A. & Gilbert, D. M. Differentiation-induced replication-timing changes are restricted to AT-rich/long interspersed nuclear element (LINE)-rich isochores. Proc. Natl Acad. Sci. USA 101, 16861–16866 (2004).
Bozhenok, L., Wade, P. A. & Varga-Weisz, P. WSTF-ISWI chromatin remodeling complex targets heterochromatic replication foci. EMBO J. 21, 2231–2241 (2002).
Taddei, A., Roche, D., Sibarita, J. B., Turner, B. M. & Almouzni, G. Duplication and maintenance of heterochromatin domains. J. Cell Biol. 147, 1153–1166 (1999).
Rountree, M. R., Bachman, K. E. & Baylin, S. B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nature Genet. 25, 269–277 (2000).
Collins, N. et al. An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nature Genet. 32, 627–632 (2002).
Fisher, D. & Mechali, M. Vertebrate HoxB gene expression requires DNA replication. EMBO J. 22, 3737–3748 (2003). This paper demonstrates that induction of HoxB genes requires only one cell cycle and occurs in a DNA replication-dependent manner, without a change in replication timing.
Cimbora, D. M. et al. Long-distance control of origin choice and replication timing in the human β-globin locus are independent of the locus control region. Mol. Cell. Biol. 20, 5581–5591 (2000).
White, E. J. et al. DNA replication-timing analysis of human chromosome 22 at high resolution and different developmental states. Proc. Natl Acad. Sci. USA 101, 17771–17776 (2004).
Dimitrova, D. S. & Gilbert, D. M. The spatial position and replication timing of chromosomal domains are both established in early G1 phase. Mol. Cell 4, 983–993 (1999). The authors demonstrate that the replication timing programme is established independently of origin specification.
Li, F. et al. The replication timing program of the Chinese hamster β-globin locus is established coincident with its repositioning near peripheral heterochromatin in early G1 phase. J. Cell Biol. 154, 283–292 (2001).
Li, F., Chen, J., Solessio, E. & Gilbert, D. M. Spatial distribution and specification of mammalian replication origins during G1 phase. J. Cell Biol. 161, 257–266 (2003).
Lin, C. M., Fu, H., Martinovsky, M., Bouhassira, E. & Aladjem, M. I. Dynamic alterations of replication timing in mammalian cells. Curr. Biol. 13, 1019–1028 (2003).
Smith, Z. E. & Higgs, D. R. The pattern of replication at a human telomeric region (16p13.3): its relationship to chromosome structure and gene expression. Hum. Mol. Genet. 8, 1373–1386 (1999).
Vyas, P. et al. Cis-acting sequences regulating expression of the human α-globin cluster lie within constitutively open chromatin. Cell 69, 781–793 (1992).
Flint, J. et al. The relationship between chromosome structure and function at a human telomeric region. Nature Genet. 15, 252–257 (1997).
Epner, E., Forrester, W. C. & Groudine, M. Asynchronous DNA replication within the human β-globin gene locus. Proc. Natl Acad. Sci. USA 85, 8081–8085 (1988).
Simon, I. et al. Developmental regulation of DNA replication timing at the human β globin locus. EMBO J. 20, 6150–6157 (2001).
Owen-Hughes, T. & Workman, J. L. Experimental analysis of chromatin function in transcription control. Crit. Rev. Eukaryot. Gene Expr. 4, 403–441 (1994).
Sims, R. J. 3rd, Belotserkovskaya, R. & Reinberg, D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18, 2437–2468 (2004).
Belotserkovskaya, R. et al. FACT facilitates transcription-dependent nucleosome alteration. Science 301, 1090–1093 (2003).
Kireeva, M. L. et al. Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription. Mol. Cell 9, 541–552 (2002).
Cavalli, G. & Paro, R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell 93, 505–518 (1998).
Cavalli, G. & Paro, R. Epigenetic inheritance of active chromatin after removal of the main transactivator. Science 286, 955–958 (1999).
Bender, W. & Fitzgerald, D. P. Transcription activates repressed domains in the Drosophila bithorax complex. Development 129, 4923–4930 (2002).
Hogga, I. & Karch, F. Transcription through the iab-7 cis-regulatory domain of the bithorax complex interferes with maintenance of Polycomb-mediated silencing. Development 129, 4915–4922 (2002).
Schwartz, B. E. & Ahmad, K. Transcriptional activation triggers deposition and removal of the histone variant H3.3. Genes Dev. 19, 804–814 (2005).
Schmitt, S., Prestel, M. & Paro, R. Intergenic transcription through a Polycomb group response element counteracts silencing. Genes Dev. 19, 697–708 (2005). This paper demonstrates that intergenic transcription through a Polycomb group response element is required for the switch from a silenced to an activated state.
Ahmad, K. & Henikoff, S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 (2002).
Henikoff, S., Furuyama, T. & Ahmad, K. Histone variants, nucleosome assembly and epigenetic inheritance. Trends Genet. 20, 320–326 (2004).
McKittrick, E., Gafken, P. R., Ahmad, K. & Henikoff, S. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl Acad. Sci. USA 101, 1525–1530 (2004). References 50,52–54 describe a pathway for replication-independent chromatin assembly through histone replacement in transcribed genomic regions.
Cawley, S. E. et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116, 499–509 (2004).
Impey, S. et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119, 1041–1054 (2004).
Yelin, R. et al. Widespread occurrence of antisense transcription in the human genome. Nature Biotechnol. 21, 379–386 (2003).
Mattick, J. S. RNA regulation: a new genetics? Nature Rev. Genet. 5, 316–323 (2004).
Navarro, P., Pichard, S., Ciaudo, C., Avner, P. & Rougeulle, C. Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromosome inactivation. Genes Dev. 19, 1474–1482 (2005).
Martens, J. A., Laprade, L. & Winston, F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429, 571–574 (2004).
Shibata, S. & Lee, J. T. Tsix transcription- versus RNA-based mechanisms in Xist repression and epigenetic choice. Curr. Biol. 14, 1747–1754 (2004).
Lipshitz, H. D., Peattie, D. A. & Hogness, D. S. Novel transcripts from the Ultrabithorax domain of the bithorax complex. Genes Dev. 1, 307–322 (1987).
Gribnau, J., Diderich, K., Pruzina, S., Calzolari, R. & Fraser, P. Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol. Cell 5, 377–386 (2000).
Ashe, H. L., Monks, J., Wijgerde, M., Fraser, P. & Proudfoot, N. J. Intergenic transcription and transinduction of the human β-globin locus. Genes Dev. 11, 2494–2509 (1997).
Drewell, R. A., Bae, E., Burr, J. & Lewis, E. B. Transcription defines the embryonic domains of cis-regulatory activity at the Drosophila bithorax complex. Proc. Natl Acad. Sci. USA 99, 16853–16858 (2002).
Bolland, D. J. et al. Antisense intergenic transcription in V(D)J recombination. Nature Immunol. 5, 630–637 (2004).
Bernstein, B. E. et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 (2005).
Rogan, D. F., Cousins, D. J. & Staynov, D. Z. Intergenic transcription occurs throughout the human IL-4/IL-13 gene cluster. Biochem. Biophys. Res. Commun. 255, 556–561 (1999).
Bae, E., Calhoun, V. C., Levine, M., Lewis, E. B. & Drewell, R. A. Characterization of the intergenic RNA profile at abdominal-A and Abdominal-B in the Drosophila bithorax complex. Proc. Natl Acad. Sci. USA 99, 16847–168452 (2002).
Ogawa, Y. & Lee, J. T. Xite, X-inactivation intergenic transcription elements that regulate the probability of choice. Mol. Cell 11, 731–743 (2003).
Rogan, D. F. et al. Analysis of intergenic transcription in the human IL-4/IL-13 gene cluster. Proc. Natl Acad. Sci. USA 101, 2446–2451 (2004).
Wilson, C. J. et al. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell 84, 235–244 (1996).
Cho, H. et al. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol. Cell. Biol. 18, 5355–5363 (1998).
Wittschieben, B. O. et al. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell 4, 123–128 (1999).
Wittschieben, B. O., Fellows, J., Du, W., Stillman, D. J. & Svejstrup, J. Q. Overlapping roles for the histone acetyltransferase activities of SAGA and elongator in vivo. EMBO J. 19, 3060–3068 (2000).
Schaft, D. et al. The histone 3 lysine 36 methyltransferase, SET2, is involved in transcriptional elongation. Nucleic Acids Res. 31, 2475–2482 (2003).
Jackson, D. A., Hassan, A. B., Errington, R. J. & Cook, P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 12, 1059–1065 (1993).
Wansink, D. G. et al. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J. Cell Biol. 122, 283–93 (1993). References 77 and 78 show that all nascent transcription occurs in distinct nuclear foci or transcription factories.
Wansink, D. G., Sibon, O. C., Cremers, F. F., van Driel, R. & de Jong, L. Ultrastructural localization of active genes in nuclei of A431 cells. J. Cell. Biochem. 62, 10–18 (1996).
Jackson, D. A., Iborra, F. J., Manders, E. M. & Cook, P. R. Numbers and organization of RNA polymerases, nascent transcripts, and transcription units in HeLa nuclei. Mol. Biol. Cell 9, 1523–1536 (1998).
Pombo, A. et al. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 18, 2241–2253 (1999).
Iborra, F. J., Pombo, A., Jackson, D. A. & Cook, P. R. Active RNA polymerases are localized within discrete transcription 'factories' in human nuclei. J. Cell Sci. 109, 1427–1436 (1996).
Grande, M. A., van der Kraan, I., de Jong, L. & van Driel, R. Nuclear distribution of transcription factors in relation to sites of transcription and RNA polymerase II. J. Cell Sci. 110, 1781–1791 (1997).
Hieda, M., Winstanley, H., Maini, P., Iborra, F. J. & Cook, P. R. Different populations of RNA polymerase II in living mammalian cells. Chromosome Res. 13, 135–144 (2005).
Osborne, C. S. et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nature Genet. 36, 1065–1071 (2004). This article provides the first demonstration that during transcription in vivo , distant genes co-localize to the same transcription factory.
Ross, I. L., Browne, C. M. & Hume, D. A. Transcription of individual genes in eukaryotic cells occurs randomly and infrequently. Immunol. Cell Biol. 72, 177–185 (1994).
Kimura, H., Sugaya, K. & Cook, P. R. The transcription cycle of RNA polymerase II in living cells. J. Cell Biol. 159, 777–782 (2002).
Levsky, J. M., Shenoy, S. M., Pezo, R. C. & Singer, R. H. Single-cell gene expression profiling. Science 297, 836–840 (2002). This paper reveals that genes are not continuously transcribed and individual cells consequently show unique patterns of gene transcription.
Vazquez, J., Belmont, A. S. & Sedat, J. W. Multiple regimes of constrained chromosome motion are regulated in the interphase Drosophila nucleus. Curr. Biol. 11, 1227–1239 (2001). Live cell analysis provides direct evidence for cell-cycle regulated changes in interphase chromatin motion.
Brown, K. E. et al. Expression of α- and β-globin genes occurs within different nuclear domains in haemopoietic cells. Nature Cell Biol. 3, 602–606 (2001).
Kosak, S. T. et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296, 158–162 (2002).
Gribnau, J., Hochedlinger, K., Hata, K., Li, E. & Jaenisch, R. Asynchronous replication timing of imprinted loci is independent of DNA methylation, but consistent with differential subnuclear localization. Genes Dev. 17, 759–773 (2003).
Chambeyron, S. & Bickmore, W. A. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 18, 1119–1130 (2004).
Zink, D. et al. Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J. Cell Biol. 166, 815–825 (2004).
Carter, D., Chakalova, L., Osborne, C. S., Dai, Y. F. & Fraser, P. Long-range chromatin regulatory interactions in vivo. Nature Genet. 32, 623–626 (2002).
Tolhuis, B., Palstra, R. J., Splinter, E., Grosveld, F. & de Laat, W. Looping and interaction between hypersensitive sites in the active β-globin locus. Mol. Cell 10, 1453–1465 (2002).
Murrell, A., Heeson, S. & Reik, W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nature Genet. 36, 889–893 (2004).
Horike, S., Cai, S., Miyano, M., Cheng, J. F. & Kohwi-Shigematsu, T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nature Genet. 37, 31–40 (2005).
Spilianakis, C. G. & Flavell, R. A. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nature Immunol. 5, 1017–1027 (2004).
Blanton, J., Gaszner, M. & Schedl, P. Protein: protein interactions and the pairing of boundary elements in vivo. Genes Dev. 17, 664–675 (2003).
Farrell, C. M., West, A. G. & Felsenfeld, G. Conserved CTCF insulator elements flank the mouse and human β-globin loci. Mol. Cell. Biol. 22, 3820–3831 (2002).
Long, Q., Bengra, C., Li, C., Kutlar, F. & Tuan, D. A long terminal repeat of the human endogenous retrovirus ERV-9 is located in the 5′ boundary area of the human β-globin locus control region. Genomics 54, 542–555 (1998).
Leach, K. M. et al. Reconstitution of human β-globin locus control region hypersensitive sites in the absence of chromatin assembly. Mol. Cell. Biol. 21, 2629–2940 (2001).
Routledge, S. J. & Proudfoot, N. J. Definition of transcriptional promoters in the human β globin locus control region. J. Mol. Biol. 323, 601–611 (2002).
Palstra, R. J. et al. The β-globin nuclear compartment in development and erythroid differentiation. Nature Genet. 35, 190–194 (2003).
Patrinos, G. P. et al. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes Dev. 18, 1495–1509 (2004).
Lettice, L. A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Nobrega, M. A., Ovcharenko, I., Afzal, V. & Rubin, E. M. Scanning human gene deserts for long-range enhancers. Science 302, 413 (2003).
Kleinjan, D. A. & van Heyningen, V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am. J. Hum. Genet. 76, 8–32 (2005).
Jackson, D. A. & Cook, P. R. Transcription occurs at a nucleoskeleton. EMBO J. 4, 919–925 (1985).
Kimura, H., Tao, Y., Roeder, R. G. & Cook, P. R. Quantitation of RNA polymerase II and its transcription factors in an HeLa cell: little soluble holoenzyme but significant amounts of polymerases attached to the nuclear substructure. Mol. Cell. Biol. 19, 5383–5392 (1999).
Cook, P. R. Predicting three-dimensional genome structure from transcriptional activity. Nature Genet. 32, 347–352 (2002).
Iborra, F. J., Pombo, A., McManus, J., Jackson, D. A. & Cook, P. R. The topology of transcription by immobilized polymerases. Exp. Cell Res. 229, 167–173 (1996).
Yin, H. et al. Transcription against an applied force. Science 270, 1653–1657 (1995).
Wang, H. Y., Elston, T., Mogilner, A. & Oster, G. Force generation in RNA polymerase. Biophys. J. 74, 1186–1202 (1998).
Cook, P. R. Nongenic transcription, gene regulation and action at a distance. J. Cell Sci. 116, 4483–4491 (2003).
Velagaleti, G. V. et al. Position effects due to chromosome breakpoints that map ∼900 Kb upstream and ∼1.3 Mb downstream of SOX9 in two patients with campomelic dysplasia. Am. J. Hum. Genet. 76, 652–662 (2005).
Sawado, T., Halow, J., Bender, M. A. & Groudine, M. The β-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 17, 1009–1018 (2003).
Vieira, K. F. et al. Recruitment of transcription complexes to the β-globin gene locus in vivo and in vitro. J. Biol. Chem. 279, 50350–50357 (2004).
Hassan, A. B., Errington, R. J., White, N. S., Jackson, D. A. & Cook, P. R. Replication and transcription sites are colocalized in human cells. J. Cell Sci. 107, 425–434 (1994).
Wei, X. et al. Segregation of transcription and replication sites into higher order domains. Science 281, 1502–1506 (1998). This paper provides evidence that transcription and replication occur in spatially distinct nuclear zones during S phase.
Cook, P. Duplicating a tangled genome. Science 281, 1466–1467 (1998).
Misteli, T. The concept of self-organization in cellular architecture. J. Cell Biol. 155, 181–185 (2001).
Perry, P. et al. A dynamic switch in the replication timing of key regulator genes in embryonic stem cells upon neural induction. Cell Cycle 3, 1645–1650 (2004).
Henikoff, S. Histone modifications: combinatorial complexity or cumulative simplicity? Proc. Natl Acad. Sci. USA 102, 5308–5309 (2005).
Marshall, W. F. et al. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr. Biol. 7, 930–939 (1997).
Gerlich, D. et al. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell 112, 751–764 (2003).
Caron, H. et al. The human transcriptome map: clustering of highly expressed genes in chromosomal domains. Science 291, 1289–1292 (2001).
Lercher, M. J., Urrutia, A. O. & Hurst, L. D. Clustering of housekeeping genes provides a unified model of gene order in the human genome. Nature Genet. 31, 180–183 (2002).
Versteeg, R. et al. The human transcriptome map reveals extremes in gene density, intron length, GC content, and repeat pattern for domains of highly and weakly expressed genes. Genome Res. 13, 1998–2004 (2003).
Acknowledgements
The authors would like to thank A. Horton, P. Varga-Wiesz, W. Reik and A. Corcoran for helpful discussions. We are also grateful to S. Gasser for the yeast chromatin dynamics movie and D. Dimitrova for DNA replication micrographs. J.A.M. is supported by a Natural Sciences and Engineering Research Council of Canada postdoctoral fellowship. P.F. is a Senior Fellow of the Medical Research Council (MRC) and is supported by the MRC and Biotechnology and Biological Sciences Research Council, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- NUCLEAR LAMINA
-
A scaffold of proteins — mainly lamin A/C and B — that are predominantly found in the nuclear periphery associated with the inner surface of the nuclear membrane.
- NUCLEOLUS
-
A highly organized nuclear organelle that is the site of ribosomal RNA processing and ribosome assembly.
- NUCLEAR MATRIX
-
A three-dimensional filamentous protein network in the nucleus that remains intact after high salt extraction.
- LONG INTERSPERSED NUCLEAR ELEMENTS
-
A class of repeat sequences.
- REPLICATION ORIGINS
-
DNA sequences at which DNA replication is initiated.
- POLYCOMB GROUP
-
A class of proteins — originally described in Drosophila melanogaster — that maintain the stable and heritable repression of several genes, including the homeotic genes.
Rights and permissions
About this article
Cite this article
Chakalova, L., Debrand, E., Mitchell, J. et al. Replication and transcription: Shaping the landscape of the genome. Nat Rev Genet 6, 669–677 (2005). https://doi.org/10.1038/nrg1673
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg1673
This article is cited by
-
Nanoscopic subcellular imaging enabled by ion beam tomography
Nature Communications (2021)
-
Integrating epigenomic data and 3D genomic structure with a new measure of chromatin assortativity
Genome Biology (2016)
-
Detection of RNA-DNA association by a proximity ligation-based method
Scientific Reports (2016)
-
Characterization of the HIV-1 RNA associated proteome identifies Matrin 3 as a nuclear cofactor of Rev function
Retrovirology (2011)
-
Regional regulation of transcription in the chicken genome
BMC Genomics (2010)