Key Points
-
The tagging of proteins with ubiquitin and their subsequent degradation by the 26S proteasome provides a highly regulated proteolysis system that is necessary in all eukaryotes.
-
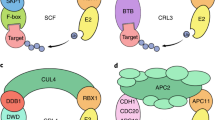
The ubiquitylation of a target protein involves the sequential action of E1, E2 and E3 enzymes; E3 ubiquitin ligase provides target specificity.
-
Plants use ubiquitin and the 26S proteasome to control a wide variety of developmental processes through the degradation of key cellular regulators.
-
Approximately 5% of the Arabidopsis thaliana genome encodes components of the ubiquitin/26S proteasome system.
-
We discuss the roles of ubiquitin in the regulation of floral development, responses to plant hormones and to pathogens, and in the regulation of photomorphogenesis.
-
The COP9 signalosome (CSN) is intimately connected to the ubiquitin/26S proteasome system and might regulate the activity of SCF-type (and possibly other) E3 ligases.
-
Recent advances, particularly in the fields of hormone signalling and responses to pathogens, have shown just how frequently plants use ubiquitin to regulate cellular processes and indicate that ubiquitin and the 26S proteasome act as regulators in many more, as yet unidentified, plant developmental processes.
Abstract
A tightly regulated and highly specific system for the degradation of individual proteins is essential for the survival of all organisms. In eukaryotes, this is achieved by the tagging of proteins with ubiquitin and their subsequent recognition and degradation by the 26S proteasome. In plants, genetic analysis has identified many genes that regulate developmental pathways. Subsequent analysis of these genes has implicated ubiquitin and the 26S proteasome in the control of diverse developmental processes, and indicates that proteolysis is a crucial regulatory step throughout the life cycle of plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tanaka, K. Molecular biology of proteasomes. Mol. Biol. Rep. 21, 21–26 (1995).
Hochstrasser, M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradtion. Curr. Biol. 7, 215–223 (1995).
Vierstra, R. D. Proteolysis in plants: mechanisms and functions. Plant Mol. Biol. 32, 275–302 (1996).
Vierstra, R. D. The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci. 8, 135–142 (2003).
Hellmann, H. & Estelle, M. Plant development: regulation by protein degradation. Science 297, 793–797 (2002).
Frugis, G. & Chua, N. H. Ubiquitin-mediated proteolysis in plant hormone signal transduction. Trends Cell Biol. 12, 308–311 (2002).
Pickart, C. M. Mechansims underlysing ubiquitination. Ann. Rev. Biochem. 70, 503–533 (2001).
Bachmir, A., Novatchkova, M., Potushak, T. & Eisenhaber, F. Ubiquitylation in plants: a post-genic look at post-translational modification. Trends Plant Sci. 6, 463–470 (2001).
Aguilar, R. C. & Wendland, B. Ubiqutin: not just for proteasomes anymore. Curr. Opin. Cell Biol. 15, 184–190 (2003).
Conaway, R. C., Brower, C. S. & Weliky-Conaway, J. Emerging roles of ubiquitin in transcriptional regulation. Science 296, 1254–1258 (2002).
Weissmann, A. M. Themes and variations on ubiquitylation. Nature Rev. Mol. Cell Biol. 2, 169–178 (2001).
Serino, G. & Deng, X. W. The COP9 signalosome: regulating plant development through the control of proteolysis. Ann. Rev. Plant Biol. 54, 165–182 (2003).
Bech-Otschir, D., Seeger, M. & Dubiel, W. The COP9 signalosome: at the interface between signal transduction and ubiquitin dependent proteolysis. J. Cell Sci. 115, 467–473 (2002).
Schwechheimer, C. & Deng, X. W. COP9 signalosome revisited; a novel mediator of protein degradation. Trends Cell Biol. 11, 420–426 (2001).
Peng, Z. et al. Evidence for a physical association of the COP9 signalosome, the proteasome, and specific SCF E3 ligases in vivo. Curr. Biol. 13, R504–R505 (2003).
Lyapina, S. et al. Promotion of NEDD8-CUL1 conjugate cleavage by COP9 signalosome. Science 292, 1382–1385 (2001).
Schwechheimer, C. et al. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292, 1379–1382 (2001). Shows that reduced CSN function leads to a loss of auxin responses similar to that seen in mutants in SCFTIR1. They also show that the CSN SCFTIR1 interact in vivo and that the CSN is required for the degradation of a putative target of SCFTIR1, indicating the importance of the CSN in regulating SCF-type E3 ligases.
Cope, G. A. et al. Role of predicted metalloprotease motif of JAB1/Csn5 in cleavage of Nedd8 from Cul1. Science 298, 608–611 (2002). The first demonstration that the Jab1/Csn5 subunit of the CSN shows NEDD8 isopeptidase activity associated with its metalloenzyme JAMM domain.
Leyser, H. M. O. et al. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin activating enzyme E1. Nature 364, 161–164 (1993).
del Pozo, J. C., Timpte, C., Tan, S., Callis, J. & Estelle, M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 280, 1760–1763 (1998).
Schwechheimer, C., Serino, G. & Deng, X. W. Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14, 2553–2563 (2002). Shows the role of the CSN in regulating many SCF E3 ligases in plants.
Estelle, M. Proteases and cellular regulation in plants. Curr. Opin. Plant Biol. 4, 254–260 (2001).
Adam, Z. Chloroplast proteases; possible regulators of gene expression? Biochimie 82, 647–654 (2000).
Palma, J. M. et al. Plant proteases, protein degradtion, and oxidative stress; role of peroxisomes. Plant Physiol. Biochem. 40, 521–530 (2002).
Adam, Z. & Clarke, A. K. Cutting edge of chloroplast proteolysis. Trends Plant Sci. 7, 451–456 (2002).
Woo, R. H. et al. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13, 1779–1790 (2001).
Stirnberg, P., van de Sande, K. & Leyser, H. M. O. MAX and MAX2 control shoot lateral branching in Arabidopsis. Development 129, 1131–1141 (2002).
Schultz, T. F., Kiyosue, T., Yanovsky, M., Wada, M. & Kay, S. A. A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell 13, 2659–2670 (2001).
Millar, A. J., Carré, I. A., Strayer, C. A., Chua, N. -H. & Kay, S. A. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267, 1161–1163 (1995).
Kim, A. S. & Delaney, T. P. Arabidopsis SON1 is an F-box protein that regulates a novel induced defense response independent of both salicyclic acid and systemic acquired resistance. Plant Cell 14, 1469–1482 (2002).
Thelander, M., Fredriksson, D., Schouten, J., Hoge, H. C. & Ronne, H. Cloning by pathway activation in yeast: identification of an Arabidopsis thaliana F-box protein that can turn on glucose repression. Plant Mol. Biol. 49, 69–79 (2002).
Ingram, G. C. et al. Dual role for fimbriata in regulating floral homeotic genes and cell division in Antirrhinum. EMBO J. 16, 6521–6534 (1997).
Karlowski, W. M. & Hirsch, A. M. The overexpression of an alfalfa RING-H2 gene induces pleiotrpic effects on plant growth and development. Plant Mol. Biol. 52, 121–133 (2003).
Stone, S. L., Anderson, E. M., Mullen, R. T. & Goring, D. R. ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell 15, 885–898 (2003). Demonstrates that, in the presence of incompatible pollen, the E3 ligase ARC1 shuttles between the nucleus and the cytosol and promotes the protein degradation events that lead to pollen rejection.
Downes, B. P., Stupar, R. M., Gingerich, D. J. & Vierstra, R. D. The HECT ubiquitin-protein ligase (UPL) family in Arabidopsis; UPL3 has a specific role in trichome development. Plant J. 35, 729–742 (2003). Describes analysis of knockout lines for the Arabidopsis HECT-domain proteins UPL3 and UPL4. Shows that mutations in UPL3 lead to defects in trichome development and that UPL3 is allelic to the previously identified kaktus mutant.
Irish, V. F. Patterning the flower. Dev. Biol. 209, 211–220 (1999).
Yanofsky, M. F. Floral meristems to floral organs; genes controlling early events in Arabidopsis flower development. Ann. Rev. Plant Physiol. Plant Mol. Biol. 46, 167–188 (1995).
Coen, E. S. & Meyerowitz, E. M. The war of the whorls; genetic interactions controlling flower development. Nature 353, 31–37 (1991).
Haughn, G. W. & Somerville, C. R. Genetic control of morphogenesis in Arabidopsis. Dev. Genet. 9, 73–89 (1988).
Riechmann, J. L. & Meyerowitz, E. MADS domain proteins in plant development. J. Biol. Chem. 378, 1079–1101 (1997).
Goto, K. & Meyerowitz, E. M. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 8, 1548–1560 (1994).
Jack, T., Brockman, L. L. & Meyerowitz, E. M. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68, 683–697 (1992).
Jack, T., Fox, G. L. & Meyerowitz, E. M. Arabidopsis homeotic gene APETALA3 ectopic expression: transcriptional and posttranscriptional regulation determine floral organ identity. Cell 76, 703–716 (1994).
Krizek, B. A. & Meyerowitz, E. M. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22 (1996).
Levin, J. Z. & Meyerowitz, E. M. UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7, 529–548 (1995).
Wilkinson, M. D. & Haughn, G. W. UNUSUAL FLORAL ORGANS controls meristem identity and organ primordial fate in Arabidopsis. Plant Cell 7, 1485–1499 (1995).
Lee, I., Wolfe, D. S., Nilsson, O. & Weigel, D. A. LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr. Biol. 7, 95–104 (1997).
Zhao, D., Yu, Q., Chen, M. & Ma, H. The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Development, 128, 2735–2746 (2001).
Samach, A. et al. The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J. 20, 433–445 (1999).
Wang, X. et al. The COP9 signalosome interacts with SCFUFO and participates in Arabidopsis flower development. Plant Cell 15, 1071–1082 (2003). Shows that the CSN is highly enriched in flowers and that plants with reduced CSN function show defects in floral development. It also shows that the F-box protein, UFO, forms part of an SCF-type E3 ligase that associates with the CSN in vivo.
Peng, Z., Serino, G. & Deng, X. W. Molecular characterization of subunit 6 of the COP9 signalosome and its role in multifaceted developmental processes in Arabidopsis. Plant Cell 13, 2393–2407 (2001).
Johri, M. M. & Mitra, D. Action of plant hormones. Curr. Sci. 80, 199–205 (2001).
Vogler, H. & Kuhlemeier, C. Simple hormones but complex signaling. Curr. Opin. Plant Biol. 6, 51–56 (2003).
Leyser, O. Molecular genetics of auxin signaling. Ann. Rev. Plant Biol. 53, 377–398 (2002).
Rogg, L. E. & Bartel, B. Auxin signaling: derepression through regulated proteolysis. Dev. Cell 1, 595–604 (2001).
del Pozo, J. C. & Estelle, M. Function of the ubiquitin-proteasome pathway in auxin response. Trends Plant Sci. 4, 107–112 (1999).
Richards, D. E., King, K. E., Ait-ali, T. & Harberd, N. P. How gibberellin regulates plant growth and development: a molecular genetic analysis of giberellin signaling. Ann. Rev. Plant Physiol. Plant Mol. Biol. 52, 67–88 (2001).
Koornneef, M. & van der Veen, J. H. Induction and analysisof gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 58, 257–263 (1980).
Sun, T., Goodman H. M. & Ausubel, F. M. Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell 4, 119–128 (1992).
Fath, A., Bethke, P., Lonsdale, J., Meza-Romero, R. & Jones, R. Programmed cell death in cereal aleurone. Plant Mol. Biol. 44, 255–266 (2000).
Silverstone, A. L., Ciampaglio, C. N. & Sun, T. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10, 155–169 (1998).
Silverstone, A. L. et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13, 1555–1565 (2001).
Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M. & Matsuoka, M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14, 57–70 (2002).
Fu, X. et al. Gibberellin-mediated proteasome-dependent degradtion of the barley DELLA protein SLN1 repressor. Plant Cell 14, 3191–3200 (2002).
Sasaki, A. et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299, 1896–1989 (2003). Demonstrates that the GA-insensitive mutant gid2 of rice is caused by a mutation in an F-box protein that forms part of an SCF-type E3 ligase. They also show that degradation of SLR1, a repressor of GA signalling, involves SCFGID2 and is controlled through GA-dependent phosophorylation of SLR1.
McGinnis, K. M. et al. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15, 1120–1130 (2003). Identifies the Arabidopsis SLEEPY gene, a positive regulator of GA responses. SLEEPY is an F-box protein that forms an SCF E3 ligase and participates in the degradation of the repressor of GA signalling RGA in response to GA.
Wang, K. L., Li, H. & Ecker, J. R. Ethylene biosynthesis and siganling networks. Plant Cell Suppl. 14, S131–S151 (2002).
Yanagisawa, S., Yoo, S. D. & Sheen, J. Differential regulation of EIN3 stability by glucose and ethylene signaling in plants. Nature 425, 521–525 (2003). Demonstrates that EIN3, a crucial regulator of ethylene signalling, is stabilized by ethylene. By contrast, addition of glucose causes the degradation of EIN3 in a proteasome-dependent manner, indicating that EIN3 is a convergence point between ethylene and sugar signalling.
Wang, Z. Y. et al. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410, 380–383 (2001).
Li, J. & Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Science 288, 2360–2363 (1997).
Wang, Z. Y. et al. Nuclear-localised BZR1 mediates brassionosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2, 505–513 (2002).
He, J. -X., Gendron, J. M., Yang, Y. & Wang, Z. -Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 10185–10190 (2002).
Lopez-Molina, L., Mongrand, S. & Chua, N. H. A post-germination developmental arrest checkpont is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl Acad. Sci. USA 32, 1–12 (2001). Describes the identification of ABI5, a transcription factor that mediates responses to ABA. They also show that ABI5 is regulated at the level of protein degradation and requires the action of the 26S proteasome and the ABI5 binding protein, AFP.
Lopez-Molina, L., Mongrand, S., Kinoshita, N. & Chua, N. H. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 17, 410–418 (2003).
Zhang, S. & Klessig, D. F. MAPK cascades in plant defense signaling. Trends Plant Sci. 6, 520–527 (2001).
Dong, X. N. Genetic dissection of systemic acquired resistance. Curr. Opin. Plant Biol. 4, 309–314 (2001).
McDowell, J. M. & Dangl, J. L. Signal transduction in the plant immune response. Trends Biochem. Sci. 25, 79–82 (2000).
Shirasu, K. & Schulze-Lefert, P. Regulators of cell death in disease resistance. Plant Mol. Biol. 44, 371–385 (2000).
Dong, X. & Sa, J. A. Ethylene and disease resistance in plants. Curr. Opin. Plant Biol. 1, 316–323 (1988).
Hammond-Kosack, K. E. & Parker, J. E. Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr. Opin. Biotechnol. 14, 177–193 (2003).
Dangl, J. L. & Jones, J. D. G. Plant pathogens and integrated defense responses to infection. Nature 411, 826–833 (2001).
Shirasu, K. et al. A novel class of eukaryotic zinc-finger binding protein is required for disease resistance signaling in barley and development in C. elegans. Cell 99, 355–366 (1999).
Azevedo, C. et al. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076 (2002). Shows that SGT1 is an interacting partner of RAR1, an essential component of MLA12-mediated resistance against powdery mildew in barley. Single-cell RNAi assays showed that SGT1 is also a crucial component of the resistance pathway.
Austin, M. J. et al. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295, 2077–2080 (2002). Describes a forward mutant screening in Arabidopsis for loss of resistance against downy mildew that identified SGT1b as a vital component of resistance.
Peart, J. R. et al. Ubiquitin ligase associated protein SGT1 is required for host and non-host disease resistance in plants. Proc. Natl Acad. Sci. USA 99, 10865–10869 (2002).
Shirasu, K. & Schulze-Lefeft, P. Complex formation, promiscuity and multi-functionality: protein interactions in disease resistance pathways. Trends Plant Sci. 8, 252–258 (2003).
Takahashi, A., Casais, C., Ichimura, K. & Shirasu, K. HSP90 interacts with RAR1 and SCT1, and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl Acad. Sci. USA 100, 11777–11782 (2003).
Dubacq, C., Guerois, R., Courbeyrette, R., Kitagawa, K. & Mann, C. Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cry1p/Cdc35p in budding yeast. Eukaryot. Cell 1, 568–582 (2002).
Liu, Y., Schiff, M., Serino, G., Deng, X. W. & Dinesh-Kumar, S. P. Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 14, 1483–1496 (2002). Shows that SKP1 and CSN components are essential for N-mediated disease resistance in Nicotiana . Both RAR1 and SGT1 associate with SCF and CSN components, indicating the functional link among these components.
Kitagawa, K., Skowyra, D., Elledge, S. J., Harper, J. W. & Hieter, P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol. Cell 4, 21–33 (1999).
Gray, W. M., Muskett, P. R., Chuang, H. -W. & Parker, J. E. Arabidopsis SGT1b is required for SCFTIR1-mediated auxin response. Plant Cell 15, 1310–1319 (2003).
Creelmann, R. A. & Mullet, J. E. Biosynthesis and action of jasmonates in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 48, 355–381 (1997).
Feys, B. J. F., Benedetti, C. E., Penfold, C. N. & Turner, J. G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6, 751–759 (1994).
Thomma, B. P. H. J. et al. Separate jasmonate-dependent and salicylate-dependent defense-repsonse pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl Acad. Sci. USA 95, 15107–15111 (1998).
McConn, M., Creelmann, R. A., Bell, E., Mullet, J. E. & Browse, J. Jasmonate is essential for insect defense. Proc. Natl Acad. Sci. USA 94, 5473–5477 (1997).
Devoto, A. et al. COI1 links jasmonate signaling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 32, 457–466 (2002)
Xu, L. et al. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14, 1919–1935 (2002).
Xie, D., Feys, B. F., James, S., Nieto-Rostro, M. & Turner, J. G. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280, 1091–1094 (1998). A landmark paper describing isolation of the COI1 gene that encodes an F-box protein, required for jasmonate, the signalling pathway in Arabidopsis.
Feng, S. et al. The COP9 signalosome interacts physically with SCFCOI1 and modulates jasmonate responses. Plant Cell 15, 1083–1094 (2003).
Sullivan, J. A. & Deng, X. W. From seed to seed; the role of photoreceptors in Arabidopsis development. Dev. Biol. 260, 289–297 (2003).
Quail, P. H. Phytochrome photosensory signaling networks. Nature Rev. Mol. Cell Biol. 3, 85–93 (2002).
Wang, H. & Deng, X. W. in The Arabidopsis Book, American Society of Plant Biologists [online], (eds Somerville, C. R. & Meyerowitz, E. M.), (cited 29 Oct 2003) <http://www.aspb.org/publications/arabidopsis> (2002).
Teppermann, J. M., Zhu, T., Chang, H. -S., Wang, X. & Quail, P. H. Multiple transcription factor genes are early targets of phytochrome A signaling. Proc. Natl Acad. Sci. USA 98, 9437–9442 (2001).
Ma, L. et al. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13, 2589–2398 (2001).
Lin, C. Blue light photoreceptors and signal transduction. Plant Cell 14, S207–S225 (2002).
Möller, S. G., Ingles, P. J. & Whitelam, G. C. The cell biology of phytochrome signaling. New Phytol. 154, 553–590 (2002).
Wei, N. & Deng, X. W. The role of the COP/DET/FUS genes in light control of Arabidopis seedling development. Plant Physiol. 112, 871–878 (1996).
Deng, X. W. et al. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gb homologous domain. Cell 71, 791–801 (1992).
Pepper, A., Delaney, T., Washburn, T., Poole, D. & Chory, J. DET1, a negative regulator of light-mediated development and gene expression in Arabidopsis, encodes a novel nuclear localized protein. Cell 78, 109–116 (1994).
Suzuki, G., Yanagawa, Y., Kwok, S. F. & Deng, X. W. Arabidopsis COP10 is a ubiquitin-conjugating enzyme variant that acts together with COP1 and the COP9 signalosome in repressing photomorphogenesis. Genes Dev. 16, 554–559 (2002). Describes the identification of COP10 , one of the essential COP/DET/FUS genes. COP10 encodes a protein showing significant homology to E2 variant proteins and indicates a possible role for non-canonical ubiquitylation in the regulation of photomorphogenesis.
Wei, N., Chamovitz, D. A. & Deng, X. W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78, 117–124 (1994).
Holm, M., Ma, L. -G., Qu, L. -J. & Deng, X. W. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Gene Dev. 16, 1247–1259 (2002).
Ballesteros, M. et al. LAF1, a myb transcription activator for phytochrome A signaling. Genes Dev. 15, 2613–2625 (2001).
Ang, L. -H. et al. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1, 213–222 (1998).
Oyama, T., Shimura, Y. & Okada, K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11, 2983–2995 (1997).
von Arnim, A. G. & Deng, X. W. Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79, 1035–1045 (1994).
Osterlund, M. T., Hardtke, C., Wei, N. & Deng, X. W. Targeted destabilization of Hy5 during light-regulated development of Arabidopsis. Nature 405, 462–466 (2000). A landmark paper that shows that the photomorphogenesis promoting factor HY5 is regulated at the level of protein degradation through the 26S proteasome and that this regulation requires the action of COP1 and other COP/DET/FUS proteins.
Saijo, Y. et al. The COP1–SPA1 interaction defines a critical step in Phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17, 2642–2647 (2003). Shows that COP1 has E3-ligase activity with respect to HY5 and that this activity can be altered by SPA1, an upstream regulator of phytochrome A signalling.
Seo, S. H. et al. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423, 995–999 (2003). This is the first demonstration of the E3 activity of COP1 on the transcription factor, LAF1, and shows that this activity can be stimulated by addition of the COP1-binding region of SPA1.
Hoffmann, R. M. & Pickart, C. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653 (1999).
Benvenuto, G., Formiggini, F., Laflamme, P., Malakhov, M. & Bowler, C. The photomorphogensis regulator DET1 binds the amino-terminal tail of histone H2B in a nucleosomal context. Curr. Biol. 12, 1529–1534 (2002).
Schroeder, D. F. et al. De-etiolated 1 and damaged DNA binding protein 1 interact to regulate Arabidopsis phoromorphogenesis. Curr. Biol. 15, 1462–1472 (2002).
Chamovitz, D. A. et al. The COP9 complex a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86, 115–121 (1996).
Quail, P. An emerging molecular map of the phytochromes. Plant Cell Environ. 20, 657–666 (1997).
Wang, H., Ma, L., Li, J., Zhao, H. & Deng, X. W. Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158 (2001).
Yang, H. -Q., Tang, R. -H. & Cashmore, A. R. The signaling mechanism if Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13, 2573–2587 (2001).
Marx, J. Ubiquitin lives up to its name. Science 297, 1792–1794 (2002).
Staub, J. M., Wei, N. & Wang, X. W. Evidence for FUS6 as a component of the nuclear localized COP9 complex in Arabidopsis. Plant Cell 8, 2047–2056 (1996).
Serino, G. et al. Characterization of the last subunit of the Arabidopsis COP9 signalosome: implications for the overall structure and origian of the complex. Plant Cell 15, 719–731 (2003).
Peng, Z., Serino, G. & Deng, X. W. A role of Arabidopsis COP9 signalosome in multifaceted developmental processes revealed by the characterization of its subunit 3. Development 128, 4277–4288 (2001).
Serino, G. et al. Arabidopsis cop8 and fus4 mutations define the same gene that encodes subunit 4 of the COP9 signalosome. Plant Cell 11, 1967–1980 (1999).
Kwok, S. F. et al. Arabidopsis homologs of a c-Jun co-activator are present both in monomeric form and in the COP9 complex, and their abundance if differentially affected by the pleiotropic cop/det/fus mutations. Plant Cell 19, 1779–1790 (1998).
Karniol, B., Malec, P. & Chamovitz, D. A. Arabidopsis FUSCA5 encodes a novel phosphoprotein that is a compoent of the COP9 complex. Plant Cell 11, 839–848 (1999).
Ferrell, K., Wilkinson, C. R. M., Dubiel, W. & Gordon, C. Regulatory subunit interactions of the 26S proteasome, a complex problem. Trends Biochem. Sci. 25, 83–88 (2000).
Wei, N. et al. The COP9 complex is conserved between plants and mammals and is related to the 26S proteasome regulatory complex. Curr. Biol. 8, 919–922 (1998).
Peng, Z. et al. Evidence for a physical association of the COP9 signalosome, the proteasome, and specific SCF E3 ligases in vivo. Curr. Biol. 12, R504–R505 (2003).
Ohi, M. D., Vander Kooi, C. W., Rosenberg, J. A., Chazin, W. J. & Gould, K. L. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nature Struct. Biol. 10, 250–255 (2003).
Azevedo, C., Santos-Rosa, M. J. & Shirasu, K. The U-box protein family in plants. Trends Plant Sci. 6, 354–358 (2001).
Gagne, J. M., Downes, B. P., Shiu, S -H., Durski, A. M. & Vierstra, R. D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 11519–11524 (2002).
Risseeuw, E. P. et al. Protein interaction of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 34, 753–767 (2003).
Acknowledgements
J.A.S. is supported by a Human Frontiers Science Program long-term fellowship. Research in K.S.'s laboratory is supported in part by grants from the Gatsby Charitable Foundation and the Biotechnology and Biological Sciences Research Council. Related research in X.W.D.'s laboratory is supported by grants from the National Institutes of Health and the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Glossary
- PROTO-ONCOGENE
-
A gene that has the potential to change into an active cancer-causing oncogene.
- ENDOCYTOSIS
-
The uptake of extracellular materials within membrane-bound vesicles by cells.
- ISOPEPTIDE BOND
-
The covalent linkage that joins amino-acid residues through an amide bond.
- THIOESTER LINKAGE
-
A non-covalent chemical bond between two proteins formed through thiolester.
- CONJUGATION
-
The addition of ubiquitin moieties to a growing polyubiquitin chain.
- SENESCENCE
-
Regulated death of an organ or a cell after its normal physiological function.
- CHELATION
-
The process by which an organic chemical bonds with metal ions and thereby removes them from solutions.
- WHORLS
-
A specific layer of flower organs that is generated through floral meristem activity.
- SEPALS
-
The protective outer layer of flower.
- STAMENS
-
The third layer of flower that bears the male gametophyte that produces pollen.
- CARPELS
-
The fourth whorl of flower that bears the female gametophyte.
- TRANSFORMATION
-
The change from one developmental pattern to another.
- APICAL DOMINANCE
-
Concentration of growth at the tip of a plant shoot, where a terminal bud exerts partial inhibition of auxiliary bud growth.
- ALEURONE CELLS
-
A cell type in cereal kernals that undergoes highly regulated cell death to release stores of minerals and nutrients to the developing embryo.
- PHENOCOPY
-
The production of a phenotype, which closely resembles a phenotype that normally results from specific gene expression or from gene mutation.
- POWDERY MILDEW
-
A group of plant diseases that are caused by the growth of fungal mycelium and the production of spores on the surface of plant tissues.
- DENEDDYLATION
-
The removal of the ubiquitin-like modifier NEDD8 (also called RUB) from a protein.
- HISTONE DEACETYLASE
-
The enzyme that removes acetyl groups from lysine residues in the DNA-binding histone group of proteins.
- SKOTOMORPHOGENIC
-
Developmental pattern followed by seedlings in the absence of light.
- HYPOCOTYLS
-
The stem of a seedling.
- COTYLEDONS
-
The leaves of a seedling formed during embryonic development.
- PHOTOMORPHOGENIC
-
Developmental pattern followed by seedlings in the presence of light.
Rights and permissions
About this article
Cite this article
Sullivan, J., Shirasu, K. & Deng, X. The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat Rev Genet 4, 948–958 (2003). https://doi.org/10.1038/nrg1228
Issue Date:
DOI: https://doi.org/10.1038/nrg1228
This article is cited by
-
Calmodulin7: recent insights into emerging roles in plant development and stress
Plant Molecular Biology (2021)
-
Aging peach palm (Bactris gasipaes Kunth) cultures lose embryogenic potential and metabolic cellular function due to continuous culture in hypoxic environments
Plant Cell, Tissue and Organ Culture (PCTOC) (2020)
-
Integration of light and hormone response during seedling establishment
Journal of Plant Biochemistry and Biotechnology (2020)
-
Cytological and transcriptome analyses reveal OsPUB73 defect affects the gene expression associated with tapetum or pollen exine abnormality in rice
BMC Plant Biology (2019)
-
Wheat TaPUB1 modulates plant drought stress resistance by improving antioxidant capability
Scientific Reports (2017)