Key Points
-
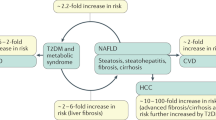
Nonalcoholic fatty liver disease (NAFLD) is present in ∼20–40% of adults in the USA, and in ∼70% and >90% of individuals worldwide with overweight or morbid obesity, respectively
-
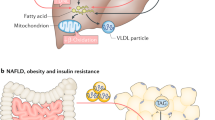
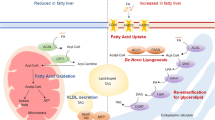
Hepatic lipid content is regulated by the complex interplay between the delivery of lipids to the liver and the processes of hepatic lipid uptake, synthesis, oxidation and secretion
-
Hepatic fat content is the strongest predictor of insulin resistance in skeletal muscle, hepatic and adipose tissues
-
Hepatokines are proteins secreted by hepatocytes that can influence metabolic processes through autocrine, paracrine and endocrine signalling
-
Hepatic steatosis induces changes in hepatokine secretion that promote insulin resistance and that negatively affect other metabolic processes
-
A better understanding of hepatokine function in hepatic steatosis will inform the prevention, diagnosis and treatment of a range of metabolic diseases, including type 2 diabetes mellitus
Abstract
Hepatic steatosis is an underlying feature of nonalcoholic fatty liver disease (NAFLD), which is the most common form of liver disease and is present in up to ∼70% of individuals who are overweight. NAFLD is also associated with hypertriglyceridaemia and low levels of HDL, glucose intolerance, insulin resistance and type 2 diabetes mellitus. Hepatic steatosis is a strong predictor of the development of insulin resistance and often precedes the onset of other known mediators of insulin resistance. This sequence of events suggests that hepatic steatosis has a causal role in the development of insulin resistance in other tissues, such as skeletal muscle. Hepatokines are proteins that are secreted by hepatocytes, and many hepatokines have been linked to the induction of metabolic dysfunction, including fetuin A, fetuin B, retinol-binding protein 4 (RBP4) and selenoprotein P. In this Review, we describe the factors that influence the development of hepatic steatosis, provide evidence of strong links between hepatic steatosis and insulin resistance in non-hepatic tissues, and discuss recent advances in our understanding of how steatosis alters hepatokine secretion to influence metabolic phenotypes through inter-organ communication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease — meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395 (2004).
Bellentani, S. et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 132, 112–117 (2000).
Luyckx, F. H. et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int. J. Obes. Relat. Metab. Disord. 22, 222–226 (1998).
Silverman, J. F. et al. Liver pathology in morbidly obese patients with and without diabetes. Am. J. Gastroenterol. 85, 1349–1355 (1990).
Leite, N. C., Salles, G. F., Araujo, A. L., Villela-Nogueira, C. A. & Cardoso, C. R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 29, 113–119 (2009).
Ludwig, J., Viggiano, T. R., McGill, D. B. & Oh, B. J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55, 434–438 (1980).
Wicklow, B. A. et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care 35, 905–910 (2012).
Yoo, H. J. & Choi, K. M. Hepatokines as a link between obesity and cardiovascular diseases. Diabetes Metab. J. 39, 10–15 (2015).
Oni, E. T. et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 230, 258–267 (2013).
Ong, J. P., Pitts, A. & Younossi, Z. M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 49, 608–612 (2008).
Yang, S. J. et al. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 96, E1325–E1329 (2011).
Michos, E. D. et al. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 200, 432–438 (2008).
Li, Q. et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J. Clin. Endocrinol. Metab. 101, 4886–4894 (2016).
Zhao, L. P. et al. Serum adropin level in patients with stable coronary artery disease. Heart Lung Circ. 24, 975–979 (2015).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014). This study provided estimates of the global prevalence of overweight and obesity in children and adults, highlighting trends over the past three decades.
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005). Using elegant tracer methodology, this study quantified the sources of hepatic and plasma lipoprotein triglycerides in patients with NAFLD.
Arner, P. & Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 25, 255–262 (2014).
Del Prato, S. et al. Insulin regulation of glucose and lipid metabolism in massive obesity. Diabetologia 33, 228–236 (1990).
Fabbrini, E. et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 134, 424–431 (2008).
Choi, Y. H. et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin. Invest. 116, 3240–3251 (2006).
Pagnon, J. et al. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 153, 4278–4289 (2012).
Chakrabarti, P. & Kandror, K. V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem. 284, 13296–13300 (2009).
Lindeboom, L. et al. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am. J. Clin. Nutr. 101, 65–71 (2015).
Turner, N. et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56, 1638–1648 (2013).
van Herpen, N. A., Schrauwen-Hinderling, V. B., Schaart, G., Mensink, R. P. & Schrauwen, P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J. Clin. Endocrinol. Metab. 96, E691–E695 (2011).
Madry, K., Lut, W., Lepert, R., Borkowska, M. & Papiernik, E. Lipid composition of plasma obtained from various parts of the vascular system of the rabbit. Acta Physiol. Pol. 27, 485–492 (1976).
Lindeboom, L. et al. Quantum coherence spectroscopy to measure dietary fat retention in the liver. JCI Insight 1, e84671 (2016).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 (2011).
Hellerstein, M. K., Schwarz, J. M. & Neese, R. A. Regulation of hepatic de novo lipogenesis in humans. Annu. Rev. Nutr. 16, 523–557 (1996).
Ameer, F., Scandiuzzi, L., Hasnain, S., Kalbacher, H. & Zaidi, N. De novo lipogenesis in health and disease. Metabolism 63, 895–902 (2014).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Uyeda, K., Yamashita, H. & Kawaguchi, T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem. Pharmacol. 63, 2075–2080 (2002).
Timlin, M. T. & Parks, E. J. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am. J. Clin. Nutr. 81, 35–42 (2005).
Degli Esposti, D. et al. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012, 387626 (2012).
Rector, R. S. et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 52, 727–736 (2010).
Szendroedi, J. et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50, 1079–1086 (2009).
Ibdah, J. A. et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128, 1381–1390 (2005).
Kim, C. S. et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr. Metab. (Lond.) 12, 33 (2015).
Aasum, E. et al. Fenofibrate modulates cardiac and hepatic metabolism and increases ischemic tolerance in diet-induced obese mice. J. Mol. Cell. Cardiol. 44, 201–209 (2008).
Park, H. S. et al. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab. J. 40, 376–385 (2016).
Demeilliers, C. et al. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology 123, 1278–1290 (2002).
Buchner, D. A. et al. Increased mitochondrial oxidative phosphorylation in the liver is associated with obesity and insulin resistance. Obesity (Silver Spring) 19, 917–924 (2011).
Koliaki, C. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21, 739–746 (2015).
Sunny, N. E., Parks, E. J., Browning, J. D. & Burgess, S. C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 14, 804–810 (2011).
Dongiovanni, P. & Valenti, L. Genetics of nonalcoholic fatty liver disease. Metabolism 65, 1026–1037 (2016).
Hussain, M. M., Shi, J. & Dreizen, P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44, 22–32 (2003).
Chen, Z., Fitzgerald, R. L., Averna, M. R. & Schonfeld, G. A targeted apolipoprotein B-38.9-producing mutation causes fatty livers in mice due to the reduced ability of apolipoprotein B-38.9 to transport triglycerides. J. Biol. Chem. 275, 32807–32815 (2000).
Cefalu, A. B. et al. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler. Thromb. Vasc. Biol. 33, 2021–2025 (2013).
Hsiao, P. J. et al. MTTP-297H polymorphism reduced serum cholesterol but increased risk of non-alcoholic fatty liver disease — a cross-sectional study. BMC Med. Genet. 16, 93 (2015).
Targher, G. et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54, 3541–3546 (2005).
Ducluzeau, P. H. et al. MRI measurement of liver fat content predicts the metabolic syndrome. Diabetes Metab. 39, 314–321 (2013).
Kotronen, A. et al. Fatty liver score and 15-year incidence of type 2 diabetes. Hepatol. Int. 7, 610–621 (2013).
Park, S. K., Seo, M. H., Shin, H. C. & Ryoo, J. H. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology 57, 1378–1383 (2013). This prospective cohort study showed that NAFLD, and particularly the more severe forms of NAFLD, is an independent risk factor for the future development of T2DM.
Zelber-Sagi, S. et al. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 33, 1406–1412 (2013).
Liu, L., Yin, X. & Morrissey, S. Global variability in diabetes mellitus and its association with body weight and primary healthcare support in 49 low- and middle-income developing countries. Diabet. Med. 29, 995–1002 (2012).
Virkamaki, A. et al. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50, 2337–2343 (2001).
Krssak, M. et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42, 113–116 (1999).
Jacob, S. et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48, 1113–1119 (1999).
Goodpaster, B. H., Thaete, F. L., Simoneau, J. A. & Kelley, D. E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46, 1579–1585 (1997).
Kelley, D. E., Thaete, F. L., Troost, F., Huwe, T. & Goodpaster, B. H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 278, E941–E948 (2000).
Unger, R. H. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144, 5159–5165 (2003).
Fabbrini, E. et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl Acad. Sci. USA 106, 15430–15435 (2009).
Korenblat, K. M., Fabbrini, E., Mohammed, B. S. & Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134, 1369–1375 (2008). This study showed that progressive increases in liver fat content are associated with progressive impairments of insulin action in the liver, skeletal muscle and adipose tissue of nondiabetic individuals with obesity.
D'Adamo, E. et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33, 1817–1822 (2010).
Linder, K. et al. Relationships of body composition and liver fat content with insulin resistance in obesity-matched adolescents and adults. Obesity (Silver Spring) 22, 1325–1331 (2014).
Gastaldelli, A. et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133, 496–506 (2007).
Koska, J. et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am. J. Clin. Nutr. 87, 295–302 (2008).
Stefan, N. et al. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 168, 1609–1616 (2008).
Zhang, H. et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci. Rep. 6, 21894 (2016).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Shimomura, I. et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6, 77–86 (2000).
Oakes, N. D., Cooney, G. J., Camilleri, S., Chisholm, D. J. & Kraegen, E. W. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 46, 1768–1774 (1997).
Samuel, V. T. et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 (2004).
Kraegen, E. W. et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40, 1397–1403 (1991). This seminal study demonstrated that hepatic steatosis and hepatic insulin resistance are early pathological features of high-fat feeding in rats.
Davis, R. C. et al. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes 59, 1616–1625 (2010).
Kim, J. K. et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl Acad. Sci. USA 98, 7522–7527 (2001).
Linden, D. et al. Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J. 20, 434–443 (2006).
Neschen, S. et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2, 55–65 (2005).
An, J. et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat. Med. 10, 268–274 (2004).
Turpin, S. M. et al. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 54, 146–156 (2011).
Savage, D. B. et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116, 817–824 (2006).
Choi, C. S. et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 (2007).
Monetti, M. et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 (2007).
Wu, J. W. et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54, 122–132 (2011).
Jacobs, R. L. et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 285, 22403–22413 (2010).
Sun, Z. & Lazar, M. A. Dissociating fatty liver and diabetes. Trends Endocrinol. Metab. 24, 4–12 (2013).
Nagle, C. A., Klett, E. L. & Coleman, R. A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 50 (Suppl.), S74–S79 (2009).
Seppala-Lindroos, A. et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 87, 3023–3028 (2002).
Utzschneider, K. M. & Kahn, S. E. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 91, 4753–4761 (2006).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014).
Puri, P. et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46, 1081–1090 (2007).
Kumashiro, N. et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 108, 16381–16385 (2011).
Magkos, F. et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 142, 1444–1446.e2 (2012).
Chaurasia, B. & Summers, S. A. Ceramides — lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Howarth, D. L. et al. Alcohol disrupts endoplasmic reticulum function and protein secretion in hepatocytes. Alcohol Clin. Exp. Res. 36, 14–23 (2012).
Xu, L., Spinas, G. A. & Niessen, M. ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Horm. Metab. Res. 42, 643–651 (2010).
Lai, K. K., Kolippakkam, D. & Beretta, L. Comprehensive and quantitative proteome profiling of the mouse liver and plasma. Hepatology 47, 1043–1051 (2008).
Kirpich, I. A. et al. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J. Nutr. Biochem. 22, 38–45 (2011).
Thomas, A. et al. Early changes in the liver-soluble proteome from mice fed a nonalcoholic steatohepatitis inducing diet. Proteomics 12, 1437–1451 (2012).
Younossi, Z. M. et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology 42, 665–674 (2005).
Fu, S. et al. Polysome profiling in liver identifies dynamic regulation of endoplasmic reticulum translatome by obesity and fasting. PLoS Genet. 8, e1002902 (2012). This study showed that the ER from obese mouse liver exhibits a general reduction in protein synthesis, demonstrating dynamic regulation of the liver translatome by obesity.
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 22, 1078–1089 (2015).
Kaur, P. et al. iTRAQ-based quantitative protein expression profiling and MRM verification of markers in type 2 diabetes. J. Proteome Res. 11, 5527–5539 (2012).
Khan, M. S., Knowles, B. B., Aden, D. P. & Rosner, W. Secretion of testosterone-estradiol-binding globulin by a human hepatoma-derived cell line. J. Clin. Endocrinol. Metab. 53, 448–449 (1981).
Lazo, M. et al. Association between endogenous sex hormones and liver fat in a multiethnic study of atherosclerosis. Clin. Gastroenterol. Hepatol. 13, 1686–1693.e2 (2015).
Peter, A. et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes 59, 3167–3173 (2010).
Sutton-Tyrrell, K. et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111, 1242–1249 (2005).
Polyzos, S. A. et al. Sex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver disease. Hormones (Athens) 12, 405–416 (2013).
Ding, E. L., Song, Y., Malik, V. S. & Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295, 1288–1299 (2006).
Ding, E. L. et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361, 1152–1163 (2009).
Perry, J. R. et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum. Mol. Genet. 19, 535–544 (2010).
Simo, R. et al. Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology 155, 2820–2830 (2014).
Simo, R., Barbosa-Desongles, A., Hernandez, C. & Selva, D. M. IL1ß down-regulation of sex hormone-binding globulin production by decreasing HNF-4α via MEK-1/2 and JNK MAPK pathways. Mol. Endocrinol. 26, 1917–1927 (2012).
Simo, R., Barbosa-Desongles, A., Lecube, A., Hernandez, C. & Selva, D. M. Potential role of tumor necrosis factor-α in downregulating sex hormone-binding globulin. Diabetes 61, 372–382 (2012).
Selva, D. M., Hogeveen, K. N., Innis, S. M. & Hammond, G. L. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J. Clin. Invest. 117, 3979–3987 (2007).
Ye, J. et al. Low serum sex hormone-binding globulin associated with insulin resistance in men with nonalcoholic fatty liver disease. Horm. Metab. Res. 49, 359–364 (2017).
Degirolamo, C., Sabba, C. & Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 15, 51–69 (2016).
Mutanen, A. et al. Serum FGF21 increases with hepatic fat accumulation in pediatric onset intestinal failure. J. Hepatol. 60, 183–190 (2014).
Zhang, X. et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 (2008).
Chavez, A. O. et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32, 1542–1546 (2009).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005). This study identified FGF21 as a novel metabolic factor with therapeutic characteristics that are necessary for the effective treatment of diabetes mellitus.
Wente, W. et al. Fibroblast growth factor-21 improves pancreatic ß-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55, 2470–2478 (2006).
Arner, P. et al. FGF21 attenuates lipolysis in human adipocytes — a possible link to improved insulin sensitivity. FEBS Lett. 582, 1725–1730 (2008).
Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
Muise, E. S. et al. Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLoS ONE 8, e73011 (2013).
Yoon, J. C. et al. Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20, 5343–5349 (2000).
Kersten, S. et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28488–28493 (2000).
Zhu, P., Goh, Y. Y., Chin, H. F., Kersten, S. & Tan, N. S. Angiopoietin-like 4: a decade of research. Biosci. Rep. 32, 211–219 (2012).
Staiger, H., Haas, C. & Machann, J. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-δ and is of metabolic relevance in humans. Diabetes 58, 579–589 (2009).
Dijk, W. et al. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 57, 1670–1683 (2016).
Yau, M. H. et al. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J. Biol. Chem. 284, 11942–11952 (2009).
Mandard, S. et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 934–944 (2006).
Xu, A. et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl Acad. Sci. USA 102, 6086–6091 (2005).
Gao, S. et al. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 63, 3242–3252 (2014).
Ganesh Kumar, K. et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 20, 1394–1402 (2012).
Kumar, K. G. et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8, 468–481 (2008). This paper detailed the discovery of adropin as a nutrient and obesity-related hepatokine that affords protection against hepatic steatosis and insulin resistance.
Butler, A. A. et al. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 97, 3783–3791 (2012).
Sayin, O., Tokgoz, Y. & Arslan, N. Investigation of adropin and leptin levels in pediatric obesity-related nonalcoholic fatty liver disease. J. Pediatr. Endocrinol. Metab. 27, 479–484 (2014).
Gao, S. et al. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 4, 310–324 (2015).
Stefan, N. et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 57, 2762–2767 (2008).
Stefan, N. & Haring, H. U. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat. Med. 19, 394–395 (2013).
Stefan, N. et al. α2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29, 853–857 (2006). This paper described the discovery of fetuin A as a liver-secreted protein that is increased with steatosis and diabetes mellitus, consistent with a potential role of fetuin A as a link between fatty liver and insulin resistance.
Haukeland, J. W. et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur. J. Endocrinol. 166, 503–510 (2012).
Srinivas, P. R. et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol. Endocrinol. 7, 1445–1455 (1993).
Mathews, S. T. et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 51, 2450–2458 (2002).
Mathews, S. T. et al. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem. Biophys. Res. Commun. 350, 437–443 (2006).
Mori, K. et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care 29, 468 (2006).
Kantartzis, K. et al. The impact of liver fat versus visceral fat in determining categories of prediabetes. Diabetologia 53, 882–889 (2010). This study showed that liver fat, more than visceral fat, substantially increases in conjunction with worsening glycaemic control in patients at risk of T2DM.
Mukhopadhyay, S. & Bhattacharya, S. Plasma fetuin-A triggers inflammatory changes in macrophages and adipocytes by acting as an adaptor protein between NEFA and TLR-4. Diabetologia 59, 859–860 (2016).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Zhu, J. et al. Serum fetuin B level increased in subjects of nonalcoholic fatty liver disease: a case-control study. Endocrine 56, 208–211 (2017).
Best, J. D. et al. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 19, 1018–1030 (1996).
Li, C. Y. et al. Recombinant human hepassocin stimulates proliferation of hepatocytes in vivo and improves survival in rats with fulminant hepatic failure. Gut 59, 817–826 (2010).
Wu, H. T. et al. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia 59, 1732–1742 (2016).
Sreekumar, R., Rosado, B., Rasmussen, D. & Charlton, M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology 38, 244–251 (2003).
Wu, H. T. et al. The role of hepassocin in the development of non-alcoholic fatty liver disease. J. Hepatol. 59, 1065–1072 (2013).
Yamagoe, S., Mizuno, S. & Suzuki, K. Molecular cloning of human and bovine LECT2 having a neutrophil chemotactic activity and its specific expression in the liver. Biochim. Biophys. Acta 1396, 105–113 (1998).
Yamagoe, S. et al. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol. Lett. 52, 9–13 (1996).
Lan, F. et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 63, 1649–1664 (2014).
Okumura, A. et al. Increased serum leukocyte cell-derived chemotaxin 2 (LECT2) levels in obesity and fatty liver. Biosci. Trends 7, 276–283 (2013).
Chikamoto, K. et al. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem. Biophys. Res. Commun. 478, 1310–1316 (2016).
Hwang, H. J. et al. LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor-mediated JNK phosphorylation in human endothelial cells. Metabolism 64, 1175–1182 (2015).
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005).
Blaner, W. S. Retinol-binding protein: the serum transport protein for vitamin A. Endocr. Rev. 10, 308–316 (1989).
Graham, T. E. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354, 2552–2563 (2006).
Ingelsson, E. et al. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis 206, 239–244 (2009).
Haider, D. G. et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 92, 1168–1171 (2007).
Lee, J. W., Lee, H. R., Shim, J. Y., Im, J. A. & Lee, D. C. Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocr. J. 55, 811–818 (2008).
Moraes-Vieira, P. M. et al. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19, 512–526 (2014).
Kotnik, P., Fischer-Posovszky, P. & Wabitsch, M. RBP4: a controversial adipokine. Eur. J. Endocrinol. 165, 703–711 (2011).
Ma, X., Zhou, Z., Chen, Y., Wu, Y. & Liu, Y. RBP4 functions as a hepatokine in the regulation of glucose metabolism by the circadian clock in mice. Diabetologia 59, 354–362 (2016).
Motsenbocker, M. A. & Tappel, A. L. A selenocysteine-containing selenium-transport protein in rat plasma. Biochim. Biophys. Acta 719, 147–153 (1982).
Olson, G. E., Winfrey, V. P., Nagdas, S. K., Hill, K. E. & Burk, R. F. Selenoprotein P is required for mouse sperm development. Biol. Reprod. 73, 201–211 (2005).
Choi, H. Y. et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab. J. 37, 63–71 (2013).
Misu, H. et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 12, 483–495 (2010). This study identified selenoprotein P as a hepatokine that causes metabolic dysfunction.
Ko, B. J., Kim, S. M., Park, K. H., Park, H. S. & Mantzoros, C. S. Levels of circulating selenoprotein P, fibroblast growth factor (FGF) 21 and FGF23 in relation to the metabolic syndrome in young children. Int. J. Obes. (Lond.) 38, 1497–1502 (2014).
Hellwege, J. N. et al. Genetic variants in selenoprotein P plasma 1 gene (SEPP1) are associated with fasting insulin and first phase insulin response in Hispanics. Gene 534, 33–39 (2014).
Zeng, M. S. et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic. Biol. Med. 52, 1335–1342 (2012).
Misu, H. et al. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS ONE 7, e34952 (2012).
Jung, T. W. et al. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein P through the AMPK-FOXO1α pathway. PLoS ONE 8, e66529 (2013).
Berger, J. P., Akiyama, T. E. & Meinke, P. T. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 26, 244–251 (2005).
Smith, B. K. et al. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am. J. Physiol. Endocrinol. Metab. 311, E730–E740 (2016).
Harriman, G. et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl Acad. Sci. USA 113, E1796–E1805 (2016).
Crookenden, M. A. et al. Short communication: proteins from circulating exosomes represent metabolic state in transition dairy cows. J. Dairy Sci. 99, 7661–7668 (2016).
Flier, J. S., Cook, K. S., Usher, P. & Spiegelman, B. M. Severely impaired adipsin expression in genetic and acquired obesity. Science 237, 405–408 (1987).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Hartwig, S. et al. Secretome profiling of primary human skeletal muscle cells. Biochim. Biophys. Acta 1844, 1011–1017 (2014).
Wang, Y. et al. Angiopoietin-like protein 4 improves glucose tolerance and insulin resistance but induces liver steatosis in high-fat-diet mice. Mol. Med. Rep. 14, 3293–3300 (2016).
Acknowledgements
The authors would like to acknowledge the support of the National Health and Medical Research Council (NHMRC) of Australia (ID: APP1061278). M.J.W. is supported by a research fellowship from the NHMRC (ID: APP1077703).
Author information
Authors and Affiliations
Contributions
R.C.R.M. researched the data for the article. M.J.W. and R.C.R.M. contributed to the discussion of the content. M.J.W. and R.C.R.M. contributed equally to writing the article, and to reviewing and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mild hepatic steatosis
-
Characterized by the storage of excess lipid in the liver and is a component of fatty liver disease.
- Nonalcoholic steatohepatitis
-
(NASH). A form of liver disease that occurs when excess fat is present in the liver accompanied by inflammation and possibly fibrosis.
- Triglyceride lipolysis
-
The breakdown of a triglyceride molecule to produce one glycerol molecule and three fatty acid molecules.
- Hepatic portal vein
-
Blood vessel that carries blood from the gastrointestinal tract, gallbladder, pancreas and spleen to the liver.
- Fenofibrate
-
A drug of the fibrate class that is generally prescribed to patients who cannot take a statin to lower blood levels of cholesterol.
- Quercetin
-
Belongs to a group of plant pigments called flavonoids and is found in many fruits, vegetables, leaves and grains.
- Lipodystrophic mice
-
Mice with a disorder of adipose tissue characterized by a selective loss of body fat.
- Haemostasis
-
The body's response to blood vessel injury and bleeding, which involves the coordinated efforts of platelets and blood-clotting proteins to form a blood clot.
- Fibrinolysis
-
The process by which fibrin is removed from damaged blood vessels, which is important in tissue remodelling and repair after injury.
- Dyslipidaemia
-
A medical condition characterized by abnormal blood levels of lipids, including cholesterol and triglycerides.
- Euglycaemic-hyperinsulinaemic clamps
-
The 'gold-standard' technique used to measure insulin sensitivity in vivo.
- Glucose effectiveness
-
Explains how glucose promotes its own uptake into tissues and accounts for 50% of glucose clearance during an oral glucose tolerance test in normal individuals and 80% in patients with obesity who are insulin resistant.
Rights and permissions
About this article
Cite this article
Meex, R., Watt, M. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 13, 509–520 (2017). https://doi.org/10.1038/nrendo.2017.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2017.56
This article is cited by
-
Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes
Scientific Reports (2024)
-
Nutrient patterns in relation to insulin resistance and endothelial dysfunction in Iranian women
Scientific Reports (2024)
-
Exerkines and cardiometabolic benefits of exercise: from bench to clinic
EMBO Molecular Medicine (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)