Key Points
At-a-glance
-
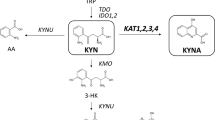
Kynurenines are the main products of tryptophan metabolism.
-
The kynurenine pathway is activated by interferon-γ and other immune-system mediators.
-
Quinolinic acid is an agonist at N-methyl-d-aspartate (NMDA) receptors, and is excitatory and neurotoxic.
-
Kynurenic acid is an antagonist at glutamate receptors and nicotinic acetylcholine (nACh) receptors, and might act at other unidentified sites.
-
Quinolinic acid, acting at NMDA receptors during brain development, could contribute to neuronal growth and synaptogenesis.
-
3-Hydroxykynurenine generates free radicals and is also neurotoxic.
-
The levels of kynurenines are elevated in disorders that involve brain damage, and might contribute to that damage. For example, quinolinic acid or 3-hydroxykynurenine levels are increased in AIDS–dementia, Huntington's disease and traumatic brain injury.
-
Kynurenic acid levels are raised in Down syndrome, neonatal asphyxia and schizophrenia.
-
As glutamate and nACh receptors are present in peripheral tissues, some peripheral disorders might be associated with altered kynurenine activity.
-
Several components of the pathway could contribute to type 2 diabetes.
-
Depletion of tryptophan inhibits cell growth and division.
-
As the predominant metabolites of tryptophan metabolic pathways also yield 5-hydroxytryptamine and melatonin, changes in the kynurenine pathway could influence the biology of all indole derivatives.
Abstract
The kynurenine pathway is the main pathway for tryptophan metabolism. It generates compounds that can modulate activity at glutamate receptors and possibly nicotinic receptors, in addition to some as-yet-unidentified sites. The pathway is in a unique position to regulate other aspects of the metabolism of tryptophan to neuroactive compounds, and also seems to be a key factor in the communication between the nervous and immune systems. It also has potentially important roles in the regulation of cell proliferation and tissue function in the periphery. As a result, the pathway presents a multitude of potential sites for drug discovery in neuroscience, oncology and visceral pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peters, J. C. Tryptophan nutrition and metabolism: an overview. Adv. Exp. Med. Biol. 294, 345–358 (1991).
Stone, T. W. & Perkins, M. N. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 72, 411–412 (1981).The original report of the neuroactive properties of quinolinic acid at NMDA receptors, and the trigger for the current widespread interest in the kynurenine pathway.
Schwarcz, R., Whetsell, W. O. Jr & Mangano, R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219, 316–318 (1983).The first paper to show directly the neurotoxic activity of quinolinic acid in the CNS.
Perkins, M. N. & Stone, T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 247, 184–187 (1982).The first report of the ability of kynurenic acid to block glutamate receptors, including those for NMDA, kainate and quisqualate (used as an AMPA-receptor agonist before the discovery of metabotropic receptors).
Thomas, S. R. & Stocker, R. Redox reactions related to IDO and tryptophan metabolism along the kynurenine pathway. Redox Rep. 4, 199–220 (1999).
Pfefferkorn, E. R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cell to degrade tryptophan. Proc. Natl Acad. Sci. USA 81, 908–912 (1984).
MacKenzie, C. R. et al. Growth inhibition of multiresistant enterococci by interferon-γ-activated human uro-epithelial cells. J. Med. Microbiol. 48, 935–941 (1999).
Carlin, J. M., Ozaki, Y., Byrne, G. I., Brown, R. R. & Borden, E. C. Interferons and IDO: role in antimicrobial and antitumour effects. Experientia 45, 535–541 (1989).
Rzeski, W., Turski, L. & Ikonomidou, C. Glutamate antagonists limit tumour growth. Proc. Natl Acad. Sci USA 98, 6372–6377 (2001).
Moffett, J. R. et al. Quinolinate immunoreactivity in experimental rat brain tumors is present in macrophages but not in astrocytes. Exp. Neurol. 144, 287–301 (1997)
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).One of the clearest links between activation of the kynurenine pathway and modulation of the physiological/immune axis, with devastating pathological consequences.
Thomas, S. R., Mohr, D. & Stocker, R. Nitric oxide inhibits IDO activity in IFN-γ primed mononuclear phagocytes. J. Biol. Chem. 269, 14457–14464 (1994).
Sekkai, D., Guittet, O., Lemaire, G., Tenu, J.-P. & Lepoivre, M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch. Biochem. Biophys. 340, 117–123 (1997).
Stone, T. W. The neuropharmacology of quinolinic acid and kynurenic acid. Pharmacol. Rev. 45, 309–379 (1993).A major review of the first decade of kynurenine research in the CNS.
Stone, T. W. The development and therapeutic potential of kynurenic acid and kynurenine derivatives for CNS neuroprotection. Trends Pharmacol. Sci. 21, 149–154 (2000).
Stone, T. W. Kynurenines in the CNS: from obscurity to clinical relevance. Progr. Neurobiol. 64, 185–218 (2001).A review of the neurobiology of kynurenine, which concentrates on the relevance of quinolinic acid in various disease states.
Bordelon, Y. M., Chesselet, M.-F., Nelson, D., Welsh, F. & Erecinska, M. Energetic dysfunction in quinolinic acid-lesioned rat striatum. J. Neurochem. 69, 1629–1693 (1997).
Baran, H. et al. Kynurenic acid influences the respiratory parameters of rat heart mitochondria. Pharmacology 62, 119–123 (2001).
Santamaria, A. et al. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. Neuroreport 12, 2693–2696 (2001)
Behan, W. M. H., McDonald, M., Darlington, L. G. & Stone, T. W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br. J. Pharmacol. 128, 1754–1760 (1999).
Kim, J. P. & Choi, D. W. Quinolinate neurotoxicity in cortical cell culture. Neuroscience 23, 423–432 (1987).
Khaspekov, L., Kida, E., Victorov, I. & Mossakowski, M. J. Neurotoxic effect induced by quinolinic acid in dissociated cell culture of mouse hippocampus. J. Neurosci. Res. 22, 150–157 (1989).
Whetsell, W. O. Jr & Schwarcz, R. Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci. Lett. 97, 271–275 (1989).
Kerr, S. J., Armati, P. J., Guillemin, G. J. & Brew, B. J. Chronic exposure of human neurones to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. AIDS 12, 355–363 (1998).
Guidetti, P. & Schwarcz, R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 11, 3857–3863 (1999).
Ghorayeb, I. et al. Simultaneous intrastriatal 6-hydroxydopamine and quinolinic acid injection. A model of early-stage striatonigral degneration. Exp. Neurol. 167, 133–147 (2001).
Behan, W. M. H. & Stone, T. W. Enhanced neuronal damage by co-administration of quinolinic acid and free radicals and protection by adenosine A2A receptor antagonists. Br. J. Pharmacol. 135, 1435–1442 (2002).
Dikranian, K. et al. Apoptosis in the developing mammalian forebrain. Neurobiol. Disease 8, 359–379 (2001).
Gurdon, J. B. & Bourillot, P.-Y. Morphogen gradient interpretation. Nature 413, 797–803 (2001).
Rezaie, P. & Male, D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microscopy Res. Tech. 45, 359–382 (1999).
McGowan, J. E. et al. Effect of dexamethasone treatment on maturational changes in the NMDA receptor in sheep brain. J. Neurosci. 20, 7424–7429 (2000).
Dong-Ruyl, L., Sawada, M. & Nakano, K. Tryptophan and its metabolite, kynurenine, stimulate expression of NGF in cultured mouse astroglial cells. Neurosci. Lett. 244, 17–20 (1998).
Heyes, M. P., Rubinow, D., Lane, C. & Markey, S. P. Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann. Neurol. 26, 275–277 (1989).A key paper, which shows the marked extent of the rise in quinolinic acid levels in the brains of patients with a major CNS disorder that is associated with cognitive dysfunction.
Espey, M. G., Moffett, J. R. & Namboodiri, M. A. A. Temporal and spatial changes of quinolinic acid immunoreactivity in the immune system of lipopolysaccharide-stimulated mice. J. Leukocyte Biol. 57, 199–206 (1995).
Namboodiri, A. M. A. et al. Increased quinolinate immunoreactivity in the peripheral blood macrocytes/macrophages from SIV-infected monkeys. J. Neurovirol. 2, 433–438 (1996).
Heyes, M. P. et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurologic disease. Brain 115, 1249–1273 (1992).An extensive study of the potential contribution of quinolinic acid to inflammatory brain disorders.
Pemberton, L. A., Kerr, S. J., Smythe, G. & Brew, B. J. Quinolinic acid production by macrophages stimulated with IFN-γ, TNF-α and IFN-α. J. Int. Cytokine Res. 17, 589–595 (1997).
Kohler, C. et al. Quinolinic acid metabolism in the rat brain. Immunohistochemical identification of 3-hydroxyanthranilic acid oxygenase and quinolinic acid phosphoribosyltransferase in the hippocampal region. J. Neurosci. 8, 975–987 (1998).
Espey, M. G., Tang, Y., Morse, H. C., Moffett, J. R. & Namboodiri, M. A. A. Localisation of quinolinic acid in the murine AIDS model of retrovirus-induced immunodeficiency: implications for neurotoxicity and dendritic cell immunopathogenesis. AIDS 10, 151–158 (1996).
Guidetti, P., Eastman, C. L. & Schwarcz, R. Metabolism of [5-3H]-kynurenine in rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J. Neurochem. 65, 2621–2632 (1995).
Guillemin, G. J. et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 78, 842–853 (2001).
Kazda, H., Taylor, N., Healy, D. & Walker, D. Maternal, umbilical and amniotic fluid concentrations of tryptophan and kynurenine after labor or cesarean section. Pediatr. Res. 44, 368–373 (1998).
Milart, P., Urbanska, E. M., Turski, W. A., Paszkowski, T. &. Sikorski, R. Intrapartum levels of endogenous glutamate antagonist kynurenic acid in amniotic fluid, umbilical and maternal blood. Neurosci. Res. Commun. 24, 173–178 (1999).
Nicholls, T., Nitsos, I. & Walker, D. W. Tryptophan metabolism in pregnant sheep: increased fetal kynurenine production in response to maternal tryptophan loading. Am. J. Obst. Gyn. 181, 1452–1460 (1999).
Henderson, G., Johnson, J. W. & Ascher, P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse NMDA receptors. J. Physiol. 430, 189–212 (1990).
Birch, P. J., Grossman, C. J. & Hayes, A. G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 154, 85–87 (1988).
Scharfman, H. E., Hodgkin, P. S., Lee, S.-C. & Schwarcz, R. Quantitative differences in the effects of de novo produced and exogenous kynurenic acid in rat brain slices. Neurosci. Lett. 274, 111–114 (1999).A study that shows that the effects of endogenously generated kynurenines are far more effective than those added exogenously, which has implications for appreciating the potential functional disturbances that could follow alterations in their endogenous concentrations.
Schwarcz, R. et al. Modulation and function of kynurenic acid in the immature rat brain. Adv. Exp. Med. Biol. 467, 113–123 (1999).
Stone, T. W. Comparison of kynurenic acid and 2-APV suppression of epileptiform activity in rat hippocampal slices. Neurosci. Lett. 84, 234–238 (1988).
Brady, R. J. & Swann, J. W. Suppression of ictal-like activity by kynurenic acid does not correlate with its efficacy as an NMDA receptor antagonist. Epilepsy Res. 2, 232–238 (1988).
Carpenedo, R. et al. Presynaptic kynrenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 13, 2141–2147 (2001).
Hilmas, C. et al. The brain metabolite kynurenic acid inhibits α7-nicotinic receptor activity and increases non-α7-nicotinic receptor expression: pathophysiological implications. J. Neurosci. 21, 7463–7473 (2001).One of the recent papers to propose an important site of action of kynurenic acid other than its blockade of glutamate receptors.
Erhardt, S., Oberg, H. & Engberg, G. Pharmacologically elevated levels of endogenous kynurenic acid prevent nicotine-induced activation of nigral dopamine neurones. Arch. Pharmacol. 363, 21–27 (2001)
Eastman, C. L. & Guilarte, T. R. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem. Res. 15, 1101–1107 (1990).
Okuda, S., Nishiyama, N., Saito, H. & Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 70, 299–307 (1998).
Chiarugi, A., Meli, E. & Moroni, F. Similarities and differences in the neuronal death processes activated by 3-hydroxykynurenine and quinolinic acid. J. Neurochem. 77, 1310–1318 (2001).
Heyes, M. P. et al. Elevated CSF quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain Res. 124, 1033–1042 (2001).
Sardar, A. M. & Reynolds, G. P. Frontal cortex indoleamin-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci. Lett. 187, 9–12 (1995).
Heyes, M. P. et al. Inter-relationships between neuroactive kynurenines, neopterin and 2-microglobulin in CSF and serum of HIV-1 infected patients. J. Neuroimmunol. 40, 71–80 (1992).
Beal, M. F. et al. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321, 168–171 (1986).
Beal, M. F., Ferrante, R. J., Swartz, K. J. & Kowall, N. W. Chronic quinolinic acid lesions in rats closely resemble Huntington's Disease. J. Neurosci. 11, 1649–1659 (1991).A detailed analysis of the neurochemical changes after quinolinic acid administration compared with the changes in a major neurodegenerative disorder — Huntington's disease.
Nicholson, L. F. B., Faull, R. L. M., Waldvogel, H. J. & Dragunow, M. GABA and GABAA receptor changes in the substantia nigra of the rat following quinolinic acid lesions in the striatum closely resemble Huntington's disease. Neuroscience 66, 507–521 (1995).
Carlock, L., Walker, P. D., Shan, Y. & Gutridge, K. Transcription of the Huntington disease gene during the quinolinic acid excitotoxic cascade. Neuroreport 6, 1121–1124 (1995).
Schwarcz, R., Okuno, E., White, R. J., Bird, E. D. & Whetsell, W. O. Jr. 3-Hydroxyanthranilate oxygenase activity is increased in the brains of Huntington disease victims. Proc. Natl Acad. Sci. USA 85, 4079–4081 (1988).
Connick, J. H., Carla, V., Moroni, F. & Stone, T. W. Increase in kynurenic acid in Huntington's disease motor cortex. J. Neurochem. 52, 985–987 (1989).
Beal, M. F. et al. Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J. Neurol. Sci. 108, 80–87 (1992).
Jauch, D. et al. Dysfunction of brain kynurenic acid metabolism in Huntington's disease: Focus on kynurenine aminotransferases. J. Neurol. Sci. 130, 39–47 (1995).
Pearson, S. J. & Reynolds, G. P. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington's disease. Neurosci. Lett. 144, 199–201 (1992).
Guidetti, P., Reddy, P. H., Tagle, D. A. & Schwarcz, R. Early kynurenergic impairment in Huntington's disease and in a transgenic animal model. Neurosci. Lett. 283, 233–235 (2000).
Hansson, O. et al. Mice expressing a Huntington's disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity. Proc. Natl Acad. Sci. USA 96, 8727–8732 (1999).
MacGibbon, G. A. et al. Immediate-early gene response to methamphetamine, haloperidol and quinolinic acid is not impaired in Huntington's disease transgenic mice. J. Neurosci. Res. 67, 372–378 (2002).
Widner, B. et al. Tryptophan degradation and immune activation in Alzheimer's disease. J. Neural Transm. 107, 343–353 (2000).
Darlington, L. G. et al. Concentrations of kynurenine pathway metabolites in patients with Huntington's disease. Soc. Neurosci. Abstr. (in the press).
Widner, B. et al. Degradation of tryptophan in neurodegenerative disorders. Adv. Exp. Med. Biol. 467, 133–138 (1999).
Baran, H., Cairns, N., Lubec, B. & Lubec, G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 58, 1891–1899 (1996).
Savvateeva, E. et al. Age-dependent memory loss, synaptic pathology and altered brain plasticity in the Drosophila mutant cardinal accumulating 3-hydroxykynurenine. J. Neural Transm. 107, 581–601 (2000).
Heyes, M. P. et al. Poliovirus induces IDO and quinolinic acid synthesis in macaque brain. FASEB J. 6, 2977–2989 (1992).
Halperin, J. J. & Heyes, M. P. Neuroactive kynurenines in Lyme borreliosis. Neurology 42, 43–50 (1992).
Sanni, L. A. et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and non-cerebral malaria. Am. J. Pathol. 152, 611–619 (1998).
Heyes, M. P. & Nowak, T. S. Jr. Delayed increases in regional brain quinolinic acid follow transient ischemia in the gerbil. J. Cereb. Blood Flow Metab. 10, 660–667 (1990).A paper that highlights the fact that raised kynurenine-pathway activity can be part of a secondary, inflammatory response to tissue damage that could exacerbate or prolong that damage.
Baratte, S. et al. Temporal and spatial changes of quinolinic acid immunoreactivity in the gerbil hippocampus following transient cerebral ischemia. Mol. Brain Res. 59, 50–57 (1998).
Blight, A. R., Leroy, E. C. & Heyes, M. P. Quinolinic acid accumulation in injured spinal cord: time course, distribution and species differences between rat and guinea-pig. J. Neurotrauma 14, 89–98 (1997).
Sinz, E. H. et al. Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 18, 610–615 (1998).
Baran, H. et al. Increased kynurenic acid in the brain after neonatal asphyxia. Life Sci. 69, 1249–1256 (2001).
Ceresoli-Borroni, G. & Schwarcz, R. Neonatal asphyxia in rats: acute effects on cerebral kynurenine metabolism. Pediatr. Res. 50, 231–235 (2001).
Dang, Y., Dale, W. E & Brown, O. R. Comparative effects of oxygen on IDO and TDO of the kynurenine pathway. Free Radical Biol. Med. 28, 615–624 (2000).
Issa, F. et al. A multidimensional approach to analysis of CSF biogenic amines in schizophrenia. II. Correlations with psychopathology. Psychiatr. Res. 52, 251–258 (1994).
Erhardt, S. et al. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 313, 96–98 (2001).
Chiarugi, A., Cozzi, A., Ballerini, C., Massacesi, L. & Moroni, F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience 102, 687–695 (2001).One of the more recent papers to address the potential role of kynurenines in a major neurological disorder — in this case, multiple sclerosis.
Maloney, A. M., St Claire Morgan, O., Widner, B., Werner, E. R. & Fuchs, D. CNS activation of the IDO pathway in human T cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J. Infect. Dis. 181, 2037–2040 (2000).
Baron, B. M. et al. Potent indole- and quinoline-containing NMDA antagonists acting at the strychnine-insensitive glycine binding site. J. Pharmacol. Exp. Ther. 262, 947–956 (1992).
Leeson, P. D. et al. Kynurenic acid derivatives — structure–activity relationships for excitatory amino acid antagonism and identification of potent and selective antagonists at the glycine site on the NMDA receptor. J. Med. Chem. 34, 1243–1252 (1991).
Harrison B. L., Baron, B. M., Cousino, D. M. & McDonald, I. A. 4-[(Carboxymethyl)oxy]- and 4-[(carboxymethyl)amino]-5,7-dichloroquinoline-2-carboxylic acid: new antagonists of the strychnine-insensitive glycine binding site on the NMDA receptor complex. J. Med. Chem. 33, 3130–3132 (1990).
Farr, R. A., Nyce, P. L. & Harrison, B. L. Heterocycle substituted propenoic acid derivatives as NMDA antagonists. Patent WO9613501 (1996).
Cugola, A. & Gavaraghi, G. Indole antagonists of excitatory amino acids. British patent GB2266091 (1993).
Jackson, P. F. et al. Synthesis and biological activity of a series of 4-aryl substituted benz[b]azepines: antagonists at the strychnine-insensitive glycine site. Bioorg. Med. Chem. Lett. 5, 3097–3100 (1995).
Boireau, A. et al. Neuroprotective effects of RPR104632, a novel antagonist at the glycine site of the NMDA receptor. Eur. J. Pharmacol. 300, 237–246 (1996).
Gottschlich, R., Leibrock, J., Noe, C., Berger, M. & Buchstaller, H.-P. Thienopyridone derivatives, their preparation and their use as NMDA-receptor antagonists. European patent EP717044 (1996).
Aloup, J. C. et al. 4-hydroxy-3-phenyl-indeno(1,2-B)pyridine-2(1H)-one derivatives as NMDA antagonists. Patent WO9602536 (1996).
Chapman, A. G. et al. Anticonvulsant activity of a novel NMDA glycine site antagonist, MDL 104,653, against kindled and sound-induced seizures. Eur. J. Pharmacol. 274, 1–3 (1995).
Kulagowski, J. J. Glycine-site NMDA antagonists: an update. Exp. Opin. Ther. Pat. 6, 1069–1079 (1996).
Takano, K. et al. Glycine site antagonists attenuate infarct size in experimental focal ischaemia: postmortem and diffusion mapping studies. Stroke 28, 1255–1263 (1997).
Harris, C. A. et al. Modulation of striatal quinolinate neurotoxicity by elevation of endogenous brain kynurenic acid. Br. J. Pharmacol. 124, 391–399 (1998).
Wu, H.-Q. et al. Kynurenergic manipulations influence excitatory synaptic function and excitotoxic vulnerability in the rat hippocampus in vivo. Neuroscience 97, 243–251 (2000).
Guidetti, F., Wu, H.-Q. & Schwarcz, R. In situ produced 7-chlorokynurenate provides protection against quinolinate and malonate-induced neurotoxicity in the rat striatum. Exp. Neurol. 163, 123–130 (2000).
Wu, H.-Q., Lee, S.-C. & Schwarcz, R. Systemic administration of 4-chlorokynurenine prevents quinolinic acid neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 390, 267–274 (2000).
Connick, J. H. et al. Nicotinylalanine increases cerebral kynurenic acid content and has anticonvulsant activity. Gen. Pharmacol. 23, 235–239 (1992).One of the first studies to show the principle that inhibition of the kynurenine pathway could increase kynurenic acid levels sufficiently to suppress neuronal overactivity and potential toxicity.
Russi, P. et al. Nicotinylalanine increases the formation of kynurenic acid in the brain and antagonizes convulsions. J. Neurochem. 59, 2076–2080 (1992).
Chiarugi, A., Carpenedo, R. & Moroni, F. Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and kynurenase. J. Neurochem. 67, 692–698 (1996).
Cozzi, R., Carpenedo, R. & Moroni, F. Kynurenine hydroxylase inhibitors reduce ischaemic brain damage: studies with (m-nitrobenzoyl)alanine and 3,4-dimethoxy-[N-4-(nitrophenyl)thiazol-2-yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global ischaemia. J. Cereb. Blood Flow Metab. 19, 771–777 (1999).An excellent example of the use of kynurenine-pathway inhibitors to prevent brain damage that is caused by ischaemia, implicating the pathway in the development of that damage.
Speciale, C. et al. (r,s)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur. J. Pharmacol. 315, 263–267 (1996).
Rover, S., Cesura, A. M., Hugenin, P., Kettler, R. & Szente, A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 40, 4378–4385 (1997).
Heidempergher, F. et al. Pyrrolo[3,2c]quinoline derivatives: a new class of kynurenine-3-hydroxylase inhibitors. Il Farmaco 54, 152–160 (1999).
Giordani, A. et al. 4-Phenyl-4-oxo-butanoic acid derivatives as inhibitors of kynurenine-3-hydroxylase. Bioorg. Med. Chem. Lett. 8, 2907–2912 (1998).
Chiarugi, A. & Moroni, F. Quinolinic acid formation in immuno-activated mice: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[N-4-(3-nitrophenyl)thiazol-2-yl]benzenesulphonamide (Ro61-8048), two potent and selective inhibitors of kynurenine hydroxylase. Neuropharmacology 38, 1225–1233 (1999).
Ross, F. C., Botting, N. P. & Leeson, P. D. Synthesis of phosphinic acid transition state analogues for the reaction catalysed by kynureninase. Bioorg. Med. Chem. Lett. 6, 2643–2646 (1996).
Fitzgerald, D. H., Muirhead, K. M. & Botting, N. G. A comparative study of the inhibition of human and bacterial kynureninase by novel bicyclic kynurenine analogues. Bioorg. Med. Chem. 9, 983–999 (2001).
Walsh, J. L., Todd, W. P., Carpenter, B. K. & Schwarcz, R. 4-Halo-3-hydroxyanthranilic acids: potent competitive inhibitors of 3-hydroxyanthranilic acid oxygenase in vitro. Biochem. Pharmacol. 42, 985–990 (1991).
Luthman, J., Radesater A.-C. & Oberg, C. Effects of the 3-hydroxyanthranilic acid analogue NCR-631 on anoxia-, IL-1β- and LPS-induced hippocampal pyramidal cell loss in vitro. Amino Acids 14, 263–269 (1998).
Forrest, C. M., Kennedy, A., Stone, T. W. & Darlington, L. G. in Proceedings of the 10th Meeting of ISTRY (ed. Costa, C. V. L.) (Plenum, New York) (in the press).
Skerry, T. M. & Genever, P. G. Glutamate signalling in non-neuronal tissues. Trends Pharmacol. Sci. 22, 174–181 (2001).
Weaver, C. D. et al. Differential expression of glutamate receptor subtypes in rat pancreatic islets. J. Biol. Chem. 271, 12977–12984 (1996).
Okamoto, H. Effect of quinaldic acid and its relatives on insulin release from isolated Langerhans islets. Acta Vitaminol. Enzymol. 29, 227–230 (1975).
Kotake, Y., Ueda, T., Mori, T., Igaki, S. & Hattori, M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid. Acta Vitaminol. Enzymol. 29, 236–240 (1975).
Hattori, M., Kotake, Y. & Kotake, Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitaminol. Enzymol. 6, 221–228 (1984).
Takeuchi, E. & Shibata, X. Kynurenine metabolism in vitamin B6 deficient rat liver after tryptophan injection. Biochem. J. 220, 693–699 (1984).
Goldstein, L. E. et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry 39, 7266–7275 (2000).
Varga, J., Yufit, T., Hitraya, T. & Brown, R. R. Control of extracellular matrix degradation by interferon-γ. The tryptophan connection. Adv. Exp. Med. Biol. 398, 143–148 (1996).
Moroni, F., Luzzi, S., Franchi-Micheli, S. & Ziletti, L. The presence of NMDA receptors for glutamic acid in the guinea-pig myenteric plexus. Neurosci. Lett. 68, 57–62 (1986).
Forrest, C. M. et al. Purine, kynurenine, and lipid peroxidation levels in inflammatory bowel disease. J. Biomed. Sci. (in the press).
Patton, A. J. et al. Expression of an NMDA type receptor by human and rat osteoblasts. Bone 22, 645–649 (1998).
Itzstein, C. et al. Specific antagonists of NMDA receptors prevent osteoclast sealing zone formation required for bone resorption. Biochem. Biophys. Res. Commun. 268, 201–209 (2000).
Gill, S. K., Mueller, R. W., McGuire, P. F. & Pulido, O. M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol. Pathol. 28, 277–284 (2000).
Goldstein, L. E. et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen perioxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry 39, 7266–7275 (2000).
Aquilina, J. A., Carver, J. A. & Truscott, R. J. W. Elucidation of a novel polypeptide cross-link involving 3-hydroxykynurenine. Biochemistry 38, 11455–11464 (1999).
Moroni, F., Lombardi, G., Moneti, G. & Aldinio, C. The excitotoxin quinolinic acid is present in the brain of several animal species and its cortical content increases during the ageing process. Neurosci. Lett. 47, 51–55 (1984).
Johnson, T. D. & Clarke, D. E. in Quinolinic acid and the Kynurenines (ed. Stone, T. W.) 213–228 (CRC Press, Boca Raton, 1989).
Charlton, K. G., Johnson, T. D., Hamed, A. T. & Clarke, D. E. Cardiovascular actions of kynuramine and 5-hydroxykynuramine in pithed rats. J. Neural Transm. 57, 199–209 (1983).
Watts, S. W., Gilbert, L. & Webb, R. C. 5HT-2(B) receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension 26, 1056–1059 (1995).
McCormack, J. K., Beitz, A. J. & Larson, A. A. Autoradiographic localization of tryptamine sites in the rat and dog CNS. J. Neurosci. 6, 94–101 (1986).
Kelly, R. W., Amato, F. & Seamark, R. F. N-Acetyl-5-methoxykynurenamine, a brain metabolite of melatonin, is a potent inhibitor of prostaglandin biosynthesis. Biochem. Biophys. Res. Commun. 121, 372–379 (1984).
Ramsay, R. R., Tan, A. K. & Weyler, W. Kinetic properties of cloned human liver monoamine oxidase A. J. Neural Transm. 41 (Suppl.), 17–26 (1994).
Franchi, A. M., Gimeno, M. F., Cardinali, D. P. & Vacas, M. I. Melatonin, 5-methoxytryptamine and some of their analogues as cyclo-oxygenase inhibitors in rat medial basal hypothalamus. Brain Res. 405, 384–388 (1987).
Worthen, D. R., et al. Endogenous indoles as novel polyamine site ligands at the NMDA receptor complex. Brain Res. 890, 343–346 (2001).
Leon, J. et al. Modification of nitric oxide synthase activity and neuronal response in rat striatum by melatonin and kynurenine derivatives. J. Neuroendocrinol. 10, 297–302 (1998).
Politi, V., De Luca, G., Gallai, V. & Comin, M. Clinical experiences with the use of indole-3-pyruvic acid. Adv. Exp. Med. Biol. 467, 227–232 (1999).
Acknowledgements
The authors' current research on kynurenines that is included in this review is supported by the NHS R&D Levy, the Peacock Trust and the Denbies Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
FlyBase
LocusLink
3-hydroxyanthranilic acid 3,4-dioxygenase
phosphoenolpyruvate carboxykinase
Medscape DrugInfo
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- SUPEROXIDE
-
A highly reactive oxygen free radical, of the formula O2−·.
- GLYB SITE
-
The allosteric receptor site for glycine on the NMDA receptor. At this site, glycine acts as an essential co-agonist for the activation of the receptor by glutamate. The site is also known as the strychnine-resistant glycine site, to distinguish it from the inhibitory glycineA receptor, which is blocked by strychnine.
- GLUCONEOGENESIS
-
The generation of glucose from non-carbohydrate sources, mainly amino acids.
- MYENTERIC PLEXUS
-
One of the two main networks of neurons that are present in the walls of the intestine; responsible for regulating the rhythm and force of its contractions and cellular secretions.
- DYSRHYTHMIAS
-
Abnormal rhythms, usually of the heart.
Rights and permissions
About this article
Cite this article
Stone, T., Darlington, L. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 1, 609–620 (2002). https://doi.org/10.1038/nrd870
Issue Date:
DOI: https://doi.org/10.1038/nrd870
This article is cited by
-
Plasma Amino Acid Profile in Children with Autism Spectrum Disorder in Southern China: Analysis of 110 Cases
Journal of Autism and Developmental Disorders (2024)
-
Endogenous Modulators of NMDA Receptor Control Dendritic Field Expansion of Cortical Neurons
Molecular Neurobiology (2023)
-
The association between tryptophan levels and postpartum mood disorders: a systematic review and meta-analysis
BMC Psychiatry (2022)
-
Dynamics of the infant gut microbiota in the first 18 months of life: the impact of maternal HIV infection and breastfeeding
Microbiome (2022)
-
Identification and characterization of the kynurenine pathway in the pond snail Lymnaea stagnalis
Scientific Reports (2022)